MEDICAL BENEFIT MANAGEMENT PROGRAM SPECIALTY PRIOR AUTHORIZATION DRUG LIST Effective March 1, 2021
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

DRUGS REQUIRING PRIOR AUTHORIZATION in the MEDICAL BENEFIT Page 1
Effective Date: 08/01/2021 DRUGS REQUIRING PRIOR AUTHORIZATION IN THE MEDICAL BENEFIT Page 1 Therapeutic Category Drug Class Trade Name Generic Name HCPCS Procedure Code HCPCS Procedure Code Description Anti-infectives Antiretrovirals, HIV CABENUVA cabotegravir-rilpivirine C9077 Injection, cabotegravir and rilpivirine, 2mg/3mg Antithrombotic Agents von Willebrand Factor-Directed Antibody CABLIVI caplacizumab-yhdp C9047 Injection, caplacizumab-yhdp, 1 mg Cardiology Antilipemic EVKEEZA evinacumab-dgnb C9079 Injection, evinacumab-dgnb, 5 mg Cardiology Hemostatic Agent BERINERT c1 esterase J0597 Injection, C1 esterase inhibitor (human), Berinert, 10 units Cardiology Hemostatic Agent CINRYZE c1 esterase J0598 Injection, C1 esterase inhibitor (human), Cinryze, 10 units Cardiology Hemostatic Agent FIRAZYR icatibant J1744 Injection, icatibant, 1 mg Cardiology Hemostatic Agent HAEGARDA c1 esterase J0599 Injection, C1 esterase inhibitor (human), (Haegarda), 10 units Cardiology Hemostatic Agent ICATIBANT (generic) icatibant J1744 Injection, icatibant, 1 mg Cardiology Hemostatic Agent KALBITOR ecallantide J1290 Injection, ecallantide, 1 mg Cardiology Hemostatic Agent RUCONEST c1 esterase J0596 Injection, C1 esterase inhibitor (recombinant), Ruconest, 10 units Injection, lanadelumab-flyo, 1 mg (code may be used for Medicare when drug administered under Cardiology Hemostatic Agent TAKHZYRO lanadelumab-flyo J0593 direct supervision of a physician, not for use when drug is self-administered) Cardiology Pulmonary Arterial Hypertension EPOPROSTENOL (generic) -

Us Anti-Doping Agency
2019U.S. ANTI-DOPING AGENCY WALLET CARDEXAMPLES OF PROHIBITED AND PERMITTED SUBSTANCES AND METHODS Effective Jan. 1 – Dec. 31, 2019 CATEGORIES OF SUBSTANCES PROHIBITED AT ALL TIMES (IN AND OUT-OF-COMPETITION) • Non-Approved Substances: investigational drugs and pharmaceuticals with no approval by a governmental regulatory health authority for human therapeutic use. • Anabolic Agents: androstenediol, androstenedione, bolasterone, boldenone, clenbuterol, danazol, desoxymethyltestosterone (madol), dehydrochlormethyltestosterone (DHCMT), Prasterone (dehydroepiandrosterone, DHEA , Intrarosa) and its prohormones, drostanolone, epitestosterone, methasterone, methyl-1-testosterone, methyltestosterone (Covaryx, EEMT, Est Estrogens-methyltest DS, Methitest), nandrolone, oxandrolone, prostanozol, Selective Androgen Receptor Modulators (enobosarm, (ostarine, MK-2866), andarine, LGD-4033, RAD-140). stanozolol, testosterone and its metabolites or isomers (Androgel), THG, tibolone, trenbolone, zeranol, zilpaterol, and similar substances. • Beta-2 Agonists: All selective and non-selective beta-2 agonists, including all optical isomers, are prohibited. Most inhaled beta-2 agonists are prohibited, including arformoterol (Brovana), fenoterol, higenamine (norcoclaurine, Tinospora crispa), indacaterol (Arcapta), levalbuterol (Xopenex), metaproternol (Alupent), orciprenaline, olodaterol (Striverdi), pirbuterol (Maxair), terbutaline (Brethaire), vilanterol (Breo). The only exceptions are albuterol, formoterol, and salmeterol by a metered-dose inhaler when used -

The Influence of Inflammation on Anemia in CKD Patients
International Journal of Molecular Sciences Review The Influence of Inflammation on Anemia in CKD Patients Anna Gluba-Brzózka 1,* , Beata Franczyk 1, Robert Olszewski 2 and Jacek Rysz 1 1 Department of Nephrology, Hypertension and Family Medicine, Medical University of Lodz, 90-549 Lodz, Poland; [email protected] (B.F.); [email protected] (J.R.) 2 Department of Geriatrics, National Institute of Geriatrics Rheumatology and Rehabilitation and Department of Ultrasound, Institute of Fundamental Technological Research, Polish Academy of Sciences, Warsaw, Poland (IPPT PAN), 02-106 Warsaw, Poland; [email protected] * Correspondence: [email protected] Received: 18 November 2019; Accepted: 19 January 2020; Published: 22 January 2020 Abstract: Anemia is frequently observed in the course of chronic kidney disease (CKD) and it is associated with diminishing the quality of a patient’s life. It also enhances morbidity and mortality and hastens the CKD progression rate. Patients with CKD frequently suffer from a chronic inflammatory state which is related to a vast range of underlying factors. The results of studies have demonstrated that persistent inflammation may contribute to the variability in Hb levels and hyporesponsiveness to erythropoietin stimulating agents (ESA), which are frequently observed in CKD patients. The understanding of the impact of inflammatory cytokines on erythropoietin production and hepcidin synthesis will enable one to unravel the net of interactions of multiple factors involved in the pathogenesis of the anemia of chronic disease. It seems that anti-cytokine and anti-oxidative treatment strategies may be the future of pharmacological interventions aiming at the treatment of inflammation-associated hyporesponsiveness to ESA. -
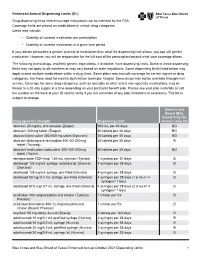
Enhanced Annual Drug List Dispensing Limits
Enhanced Annual Dispensing Limits (DL) Drug dispensing limits help encourage medication use as intended by the FDA. Coverage limits are placed on medications in certain drug categories. Limits may include: • Quantity of covered medication per prescription • Quantity of covered medication in a given time period If your doctor prescribes a greater quantity of medication than what the dispensing limit allows, you can still get the medication. However, you will be responsible for the full cost of the prescription beyond what your coverage allows. The following brand drugs, and their generic equivalents, if available, have dispensing limits. Some of these dispensing limits may not apply to all members or may vary based on state regulations. Some dispensing limits listed below may apply across multiple medications within a drug class. Some plans may exclude coverage for certain agents or drug categories, like those used for erectile dysfunction (example: Viagra). Some drugs may not be available through mail service. Coverage for some drug categories, such as specialty or other select non‑specialty medications, may be limited to a 30‑day supply at a time depending on your particular benefit plan. Please see your plan materials or call the number on the back of your ID card to verify if you are uncertain of any plan limitations or exclusions. This list is subject to change. Generic and Brand (BG), Brand Only (B), Drug (generic) strength Dispensing Limit Generic only (G) abacavir 20 mg/mL oral solution (Ziagen) 960 mL per 30 days BG abacavir 300 -

Pulmonary Delivery of Biological Drugs
pharmaceutics Review Pulmonary Delivery of Biological Drugs Wanling Liang 1,*, Harry W. Pan 1 , Driton Vllasaliu 2 and Jenny K. W. Lam 1 1 Department of Pharmacology and Pharmacy, Li Ka Shing Faculty of Medicine, The University of Hong Kong, 21 Sassoon Road, Pokfulam, Hong Kong, China; [email protected] (H.W.P.); [email protected] (J.K.W.L.) 2 School of Cancer and Pharmaceutical Sciences, King’s College London, 150 Stamford Street, London SE1 9NH, UK; [email protected] * Correspondence: [email protected]; Tel.: +852-3917-9024 Received: 15 September 2020; Accepted: 20 October 2020; Published: 26 October 2020 Abstract: In the last decade, biological drugs have rapidly proliferated and have now become an important therapeutic modality. This is because of their high potency, high specificity and desirable safety profile. The majority of biological drugs are peptide- and protein-based therapeutics with poor oral bioavailability. They are normally administered by parenteral injection (with a very few exceptions). Pulmonary delivery is an attractive non-invasive alternative route of administration for local and systemic delivery of biologics with immense potential to treat various diseases, including diabetes, cystic fibrosis, respiratory viral infection and asthma, etc. The massive surface area and extensive vascularisation in the lungs enable rapid absorption and fast onset of action. Despite the benefits of pulmonary delivery, development of inhalable biological drug is a challenging task. There are various anatomical, physiological and immunological barriers that affect the therapeutic efficacy of inhaled formulations. This review assesses the characteristics of biological drugs and the barriers to pulmonary drug delivery. -

Immunfarmakológia Immunfarmakológia
Gergely: Immunfarmakológia Immunfarmakológia Prof Gergely Péter Az immunpatológiai betegségek döntő többsége gyulladásos, és ennek következtében általában szövetpusztulással járó betegség, melyben – jelenleg – a terápia alapvetően a gyulladás csökkentésére és/vagy megszűntetésére irányul. Vannak kizárólag gyulladásgátló gyógyszereink és vannak olyanok, amelyek az immunreakció(k) bénításával (=immunszuppresszió révén) vagy emellett vezetnek a gyulladás mérsékléséhez. Mind szerkezetileg, mind hatástanilag igen sokféle csoportba oszthatók, az alábbi felosztás elsősorban didaktikus célokat szolgál. 1. Nem-szteroid gyulladásgátlók (‘nonsteroidal antiinflammatory drugs’ NSAID) 2. Kortikoszteroidok 3. Allergia-elleni szerek (antiallergikumok) 4. Sejtoszlás-gátlók (citosztatikumok) 5. Nem citosztatikus hatású immunszuppresszív szerek 6. Egyéb gyulladásgátlók és immunmoduláns szerek 7. Biológiai terápia 1. Nem-szteroid gyulladásgátlók (NSAID) Ezeket a vegyületeket, melyek őse a szalicilsav (jelenleg, mint acetilszalicilsav ‘aszpirin’ használatos), igen kiterjedten alkalmazzák a reumatológiában, az onkológiában és az orvostudomány szinte minden ágában, ahol fájdalom- és lázcsillapításra van szükség. Egyes felmérések szerint a betegek egy ötöde szed valamilyen NSAID készítményt. Szerkezetük alapján a készítményeket több csoportba sorolhatjuk: szalicilátok (pl. acetilszalicilsav) pyrazolidinek (pl. fenilbutazon) ecetsav származékok (pl. indometacin) fenoxiecetsav származékok (pl. diclofenac, aceclofenac)) oxicamok (pl. piroxicam, meloxicam) propionsav -

Analytical Approaches in Human Sports Drug Testing
Received: 13 November 2018 Accepted: 18 November 2018 DOI: 10.1002/dta.2549 ANNUAL BANNED‐ SUBSTANCE REVIEW Annual banned‐substance review: Analytical approaches in human sports drug testing Mario Thevis1,2 | Tiia Kuuranne3 | Hans Geyer1,2 1 Center for Preventive Doping Research ‐ Institute of Biochemistry, German Sport Abstract University Cologne, Cologne, Germany A number of high profile revelations concerning anti‐doping rule violations over the 2 European Monitoring Center for Emerging past 12 months have outlined the importance of tackling prevailing challenges and Doping Agents, Cologne, Germany reducing the limitations of the current anti‐doping system. At this time, the necessity 3 Swiss Laboratory for Doping Analyses, University Center of Legal Medicine, Genève to enhance, expand, and improve analytical test methods in response to the sub- and Lausanne, Centre Hospitalier Universitaire stances outlined in the World Anti‐Doping Agency (WADA) Prohibited List represents Vaudois and University of Lausanne, Epalinges, Switzerland an increasingly crucial task for modern sports drug testing programs. The ability to Correspondence improve analytical testing methods often relies on the expedient application of novel Mario Thevis, Institute of Biochemistry ‐ Center for Preventive Doping Research, information regarding superior target analytes for sports drug testing assays, drug German Sport University Cologne, Am elimination profiles, and alternative sample matrices, together with recent advances Sportpark Müngersdorf 6, 50933 Cologne, Germany. in instrumental developments. This annual banned‐substance review evaluates litera- Email: thevis@dshs‐koeln.de ture published between October 2017 and September 2018 offering an in‐depth Funding information evaluation of developments in these arenas and their potential application to Federal Ministry of the Interior, Federal Republic of Germany; Manfred‐Donike‐Insti- substances reported in WADA's 2018 Prohibited List. -

(12) Patent Application Publication (10) Pub. No.: US 2017/0172932 A1 Peyman (43) Pub
US 20170172932A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2017/0172932 A1 Peyman (43) Pub. Date: Jun. 22, 2017 (54) EARLY CANCER DETECTION AND A 6LX 39/395 (2006.01) ENHANCED IMMUNOTHERAPY A61R 4I/00 (2006.01) (52) U.S. Cl. (71) Applicant: Gholam A. Peyman, Sun City, AZ CPC .......... A61K 9/50 (2013.01); A61K 39/39558 (US) (2013.01); A61K 4I/0052 (2013.01); A61 K 48/00 (2013.01); A61K 35/17 (2013.01); A61 K (72) Inventor: sham A. Peyman, Sun City, AZ 35/15 (2013.01); A61K 2035/124 (2013.01) (21) Appl. No.: 15/143,981 (57) ABSTRACT (22) Filed: May 2, 2016 A method of therapy for a tumor or other pathology by administering a combination of thermotherapy and immu Related U.S. Application Data notherapy optionally combined with gene delivery. The combination therapy beneficially treats the tumor and pre (63) Continuation-in-part of application No. 14/976,321, vents tumor recurrence, either locally or at a different site, by filed on Dec. 21, 2015. boosting the patient’s immune response both at the time or original therapy and/or for later therapy. With respect to Publication Classification gene delivery, the inventive method may be used in cancer (51) Int. Cl. therapy, but is not limited to such use; it will be appreciated A 6LX 9/50 (2006.01) that the inventive method may be used for gene delivery in A6 IK 35/5 (2006.01) general. The controlled and precise application of thermal A6 IK 4.8/00 (2006.01) energy enhances gene transfer to any cell, whether the cell A 6LX 35/7 (2006.01) is a neoplastic cell, a pre-neoplastic cell, or a normal cell. -

2020 Medicaid Preapproval Criteria
2020 Medicaid Preapproval Criteria ABILIFY MAINTENA ................................................................................................................................................................ 10 ACTHAR HP ............................................................................................................................................................................ 11 ACTIMMUNE ......................................................................................................................................................................... 13 ADAGEN & REVCOVI ............................................................................................................................................................. 14 ADCIRCA ................................................................................................................................................................................ 15 ADEMPAS .............................................................................................................................................................................. 16 AFINITOR ............................................................................................................................................................................... 18 AFINITOR DISPERZ ................................................................................................................................................................. 19 ALDURAZYME ....................................................................................................................................................................... -

Presentation Title
The COMMANDS trial: a phase 3 study of the efficacy and safety of luspatercept versus epoetin alfa for the treatment of anemia due to Revised International Prognostic Scoring System Very Low-, Low-, or Intermediate-risk myelodysplastic syndromes in erythropoiesis stimulating agent-naive patients who require red blood cell transfusions Matteo Della Porta,1,2 Uwe Platzbecker,3 Valeria Santini,4 Guillermo Garcia-Manero,5 Rami S. Komrokji,6 Rodrigo Ito,7 Pierre Fenaux8 1Cancer Center IRCCS Humanitas Research Hospital, Milan, Italy; 2Department of Biomedical Sciences, Humanitas University, Milan, Italy; 3Medical Clinic and Policlinic 1, Hematology and Cellular Therapy, University Hospital Leipzig, Leipzig, Germany; 4Azienda Ospedaliero-Universitaria Careggi, University of Florence, Florence, Italy; 5Department of Leukemia, University of Texas MD Anderson Cancer Center, Houston, TX; 6Moffitt Cancer Center, Tampa, FL; 7Bristol Myers Squibb, Princeton, NJ; 8Service d'Hématologie Séniors, Hôpital Saint-Louis, Université Paris 7, Paris, France Presentation 2198 Presenting author disclosures M.D.P.: no conflicts of interest to disclose. 2 Introduction and objectives Introduction • Studies of epoetin alfa and darbepoetin alfa have demonstrated efficacy among patients with LR-MDS, but the patient population in which a clinically significant effect is observed may be limited1,2 • Luspatercept, a first-in-class erythroid maturation agent with a mechanism of action distinct from ESAs,3 is approved by the US FDA for the treatment of anemia failing an -

CDER List of Licensed Biological Products With
Center for Drug Evaluation and Research List of Licensed Biological Products with (1) Reference Product Exclusivity and (2) Biosimilarity or Interchangeability Evaluations to Date DATE OF FIRST REFERENCE PRODUCT DATE OF LICENSURE LICENSURE EXCLUSIVITY EXPIRY DATE INTERCHANGEABLE (I)/ BLA STN PRODUCT (PROPER) NAME PROPRIETARY NAME (mo/day/yr) (mo/day/yr) (mo/day/yr) BIOSIMILAR (B) WITHDRAWN 125118 abatacept Orencia 12/23/05 NA NA 103575 abciximab ReoPro 12/22/94 NA NA Yes 125274 abobotulinumtoxinA Dysport 04/29/09 125057 adalimumab Humira 12/31/02 NA NA 761071 adalimumab-adaz Hyrimoz 10/30/18 B 761058 adalimumab-adbm Cyltezo 08/25/17 B 761118 adalimumab-afzb Abrilada 11/15/19 B 761024 adalimumab-atto Amjevita 09/23/16 B 761059 adalimumab-bwwd Hadlima 07/23/19 B 125427 ado-trastuzumab emtansine Kadcyla 02/22/13 125387 aflibercept Eylea 11/18/11 103979 agalsidase beta Fabrazyme 04/24/03 NA NA 125431 albiglutide Tanzeum 04/15/14 017835 albumin chromated CR-51 serum Chromalbin 02/23/76 103293 aldesleukin Proleukin 05/05/92 NA NA 103948 alemtuzumab Campath, Lemtrada 05/07/01 NA NA 125141 alglucosidase alfa Myozyme 04/28/06 NA NA 125291 alglucosidase alfa Lumizyme 05/24/10 125559 alirocumab Praluent 07/24/15 103172 alteplase, cathflo activase Activase 11/13/87 NA NA 103950 anakinra Kineret 11/14/01 NA NA 020304 aprotinin Trasylol 12/29/93 125513 asfotase alfa Strensiq 10/23/15 101063 asparaginase Elspar 01/10/78 NA NA 125359 asparaginase erwinia chrysanthemi Erwinaze 11/18/11 761034 atezolizumab Tecentriq 05/18/16 761049 avelumab Bavencio 03/23/17 -

Current Challenges and Unmet Medical Needs in Myelodysplastic Syndromes
Leukemia (2021) 35:2182–2198 https://doi.org/10.1038/s41375-021-01265-7 REVIEW ARTICLE Myelodysplastic syndrome Current challenges and unmet medical needs in myelodysplastic syndromes 1,2,3 1,2,3 4 4 Uwe Platzbecker ● Anne Sophie Kubasch ● Collin Homer-Bouthiette ● Thomas Prebet Received: 4 January 2021 / Revised: 1 April 2021 / Accepted: 26 April 2021 / Published online: 28 May 2021 © The Author(s) 2021. This article is published with open access Abstract Myelodysplastic syndromes (MDS) represent a heterogeneous group of myeloid neoplasms that are characterized by ineffective hematopoiesis, variable cytopenias, and a risk of progression to acute myeloid leukemia. Most patients with MDS are affected by anemia and anemia-related symptoms, which negatively impact their quality of life. While many patients with MDS have lower-risk disease and are managed by existing treatments, there currently is no clear standard of care for many patients. For patients with higher-risk disease, the treatment priority is changing the natural history of the disease by delaying disease progression to acute myeloid leukemia and improving overall survival. However, existing treatments for MDS are generally not curative and many patients experience relapse or resistance to first-line treatment. Thus, there remains 1234567890();,: 1234567890();,: an unmet need for new, more effective but tolerable strategies to manage MDS. Recent advances in molecular diagnostics have improved our understanding of the pathogenesis of MDS, and it is becoming clear that the diverse nature of genetic abnormalities that drive MDS demands a complex and personalized treatment approach. This review will discuss some of the challenges related to the current MDS treatment landscape, as well as new approaches currently in development.