Chapter 10 Role of Anaerobic Zones and Processes In
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

LOMR)? for a One-Year Premium Refund
the Arroyo Chico and Tucson Arroyo a FIRM. You may view this tutorial at: Property owners whose buildings have been watercourses. http://www.fema.gov/media- removed from an SFHA and are now located in library/assets/documents/7984 a Zone X or a Shaded Zone X may be eligible What is a Letter of Map Revision (LOMR)? for a one-year premium refund. Your lender When does a LOMR change a FIRM? must provide you with a letter agreeing to A LOMR is an official revision to the Flood remove the requirement for flood insurance. If Insurance Rate Maps (FIRMs) issued by the LOMRs become effective once the statutory your lender refuses to send you a letter stating Federal Emergency Management Agency Technical Appeal Period is over. The that they will not require flood insurance, you (FEMA). LOMRs reflect changes to the 100- effective date is listed on the LOMR cover will not be eligible for a refund. If you do not year floodplains or Special Flood Hazard letter. have a lender, you will not be eligible for a Areas (SFHA) shown on the FIRMs. In rare refund. To learn if you are eligible, please situations, LOMRs also modify the 500-year Can I drop my flood insurance if my follow these steps: floodplain boundaries. Changes may include residence or business is removed from ARROYO CHICO FLOODPLAIN modifications to Base Flood Elevations, the floodplain by a LOMR? 1. View the revised flood maps to REMAPPING floodplain widths, and floodways. The determine if your property has been re- QUESTIONS AND ANSWERS LOMRs are issued after a floodplain has The Flood Disaster Protection Act of 1973 mapped to a Zone X or Shaded Zone been remapped due to a major flood event, and the National Flood Insurance Reform Act X. -

River Incision Into Bedrock: Mechanics and Relative Efficacy of Plucking, Abrasion and Cavitation
River incision into bedrock: Mechanics and relative efficacy of plucking, abrasion and cavitation Kelin X. Whipple* Department of Earth, Atmospheric, and Planetary Science, Massachusetts Institute of Technology, Cambridge, Massachusetts 02139 Gregory S. Hancock Department of Geology, College of William and Mary, Williamsburg, Virginia 23187 Robert S. Anderson Department of Earth Sciences, University of California, Santa Cruz, California 95064 ABSTRACT long term (Howard et al., 1994). These conditions are commonly met in Improved formulation of bedrock erosion laws requires knowledge mountainous and tectonically active landscapes, and bedrock channels are of the actual processes operative at the bed. We present qualitative field known to dominate steeplands drainage networks (e.g., Wohl, 1993; Mont- evidence from a wide range of settings that the relative efficacy of the gomery et al., 1996; Hovius et al., 1997). As the three-dimensional structure various processes of fluvial erosion (e.g., plucking, abrasion, cavitation, of drainage networks sets much of the form of terrestrial landscapes, it is solution) is a strong function of substrate lithology, and that joint spac- clear that a deep appreciation of mountainous landscapes requires knowl- ing, fractures, and bedding planes exert the most direct control. The edge of the controls on bedrock channel morphology. Moreover, bedrock relative importance of the various processes and the nature of the in- channels play a critical role in the dynamic evolution of mountainous land- terplay between them are inferred from detailed observations of the scapes (Anderson, 1994; Anderson et al., 1994; Howard et al., 1994; Tucker morphology of erosional forms on channel bed and banks, and their and Slingerland, 1996; Sklar and Dietrich, 1998; Whipple and Tucker, spatial distributions. -

STREAMS and RUNNING WATER Hydrologic Cycle •What Happens to Precipitation STREAM •River, Creek, Etc
STREAMS AND RUNNING WATER Hydrologic Cycle •What happens to precipitation STREAM •River, Creek, etc. • Banks, Bed • Confined to a channel –Except in time of flood •Floodplain •Longtitudinal Profile Headwaters, mouth Cross-profile V-shaped SHEETWASH –Unchanneled, thin sheet of flowing water – Results in Sheet Erosion Drainage Basin •Tributary • Divide • Examples: –Mississippi River – Amazon River Drainage Patterns •As seen from above • Dendritic (most common) • Radial • Trellis- in tilted or folded layered rock Factors affecting stream erosion and deposition •Velocity- –Distribution in cross-section •Gradient –Usually decreases downstream – Effect on deposition and erosion •Channel shape and roughness • Discharge- –Volume of water moving past a place in time •Usually given as cubic feet per second (cfs) –Usually increases downstream •Discharge- –Volume of water moving past a place in time •Usually given as cubic feet per second (cfs) –Usually increases downstream American River Discharge •In Sacramento: • Average 3,741 c.f.s. • Usual Range: –Winter 9,000 cfs – Last drought 250 cfs •1986 flood 130,000 cfs Stream Erosion •Hydraulic action • Solution- Dissolves rock • Abrasion (by sand and gravel) –Potholes •Deepens valley by eroding river bed • Lateral erosion of banks on outside of curves Transportation of Sediment •Bed load • Suspended load • Dissolved load Deposition •Bars- –moved in floods – deposited as water slows – Placer Deposits (gold, etc.) – Braided Streams •heavy load of sand and gravel • commonly from glacier outwash streams -
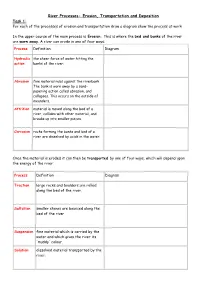
River Processes- Erosion, Transportation and Deposition Task 1: for Each of the Processes of Erosion and Transportation Draw a Diagram Show the Process at Work
River Processes- Erosion, Transportation and Deposition Task 1: For each of the processes of erosion and transportation draw a diagram show the process at work In the upper course of the main process is Erosion. This is where the bed and banks of the river are worn away. A river can erode in one of four ways: Process Definition Diagram Hydraulic the sheer force of water hitting the action banks of the river: Abrasion fine material rubs against the riverbank The bank is worn away by a sand- papering action called abrasion, and collapses. This occurs on the outside of meanders. Attrition material is moved along the bed of a river, collides with other material, and breaks up into smaller pieces. Corrosion rocks forming the banks and bed of a river are dissolved by acids in the water. Once the material is eroded it can then be transported by one of four ways, which will depend upon the energy of the river: Process Definition Diagram Traction large rocks and boulders are rolled along the bed of the river. Saltation smaller stones are bounced along the bed of the river Suspension fine material which is carried by the water and which gives the river its 'muddy' colour. Solution dissolved material transported by the river. In the middle and lower course, the land is much flatter, this means that the river is flowing more slowly and has much less energy. The river starts to deposit (drop) the material that it has been carry Deposition Challenge: Add labels onto the diagram to show where all of the processes could be happening in the river channel. -

Drainage Net
Drainage Net GEOLOGICAL SURVEY PROFESSIONAL f APER 282-A Ephemeral Streams Hydraulic Factors and Their Relation to the Drainage Net By LUNA B. LEOPOLD and JOHN P. MILLER PHYSIOGRAPHIC AND HYDRAULIC STUDIES OF RIVERS GEOLOGICAL SURVEY PROFESSIONAL PAPER 282-A UNITED STATES GOVERNMENT PRINTING OFFICE, WASHINGTON : 1956 UNITED STATES DEPARTMENT OF THE INTERIOR CECIL D. ANDRUS, Secretary GEOLOGICAL SURVEY H. William Menard, Director Fin* printing 19M Second printing 1K9 Third printing 1MB For sale by the Branch of Distribution, U.S. Geological Survey, 1200 South Eads Street, Arlington, VA 22202 CONTENTS Page Page Symbols. __________________________________________ iv Equations relating hydraulic and physiographic Abstract. __________________________________________ 1 factors....________-_______.__-____--__---_-_ 19 Introduction and acknowledgments ___________________ 1 Some relations of hydraulic and physiographic factors to Geographic setting and basic measurements ____________ 2 the longitudinal profile.___________________________ 24 Measurement of hydraulic variables in ephemeral streams, 4 Channel roughness and particle size.______________ 24 General features of flow _________________________ 4 Effect of change of particle size and velocity on Problems of measurement._______________________ 6 stream gradient.____.__._____-_______-____--__ 26 Changes of width, depth, velocity, and load at indi Equilibrium in ephemeral streams _____________________ 28 vidual channel cross sections ___________________ 7 Mutual adjustment of hydraulic factors._____.._._ -

Experiments on Patterns of Alluvial Cover and Bedrock Erosion in a Meandering Channel Roberto Fernández1, Gary Parker1,2, Colin P
Earth Surf. Dynam. Discuss., https://doi.org/10.5194/esurf-2019-8 Manuscript under review for journal Earth Surf. Dynam. Discussion started: 27 February 2019 c Author(s) 2019. CC BY 4.0 License. Experiments on patterns of alluvial cover and bedrock erosion in a meandering channel Roberto Fernández1, Gary Parker1,2, Colin P. Stark3 1Ven Te Chow Hydrosystems Laboratory, Department of Civil and Environmental Engineering, University of Illinois at 5 Urbana-Champaign, Urbana, IL 61801, USA 2Department of Geology, University of Illinois at Urbana-Champaign, Urbana, IL 61801, USA 3Lamont Doherty Earth Observatory, Columbia University, Palisades, NY 10964, USA Correspondence to: Roberto Fernández ([email protected]) Abstract. 10 In bedrock rivers, erosion by abrasion is driven by sediment particles that strike bare bedrock while traveling downstream with the flow. If the sediment particles settle and form an alluvial cover, this mode of erosion is impeded by the protection offered by the grains themselves. Channel erosion by abrasion is therefore related to the amount and pattern of alluvial cover, which are functions of sediment load and hydraulic conditions, and which in turn are functions of channel geometry, slope and sinuosity. This study presents the results of alluvial cover experiments conducted in a meandering channel flume of high fixed 15 sinuosity. Maps of quasi-instantaneous alluvial cover were generated from time-lapse imaging of flows under a range of below- capacity bedload conditions. These maps were used to infer patterns -

COLD-BASED GLACIERS in the WESTERN DRY VALLEYS of ANTARCTICA: TERRESTRIAL LANDFORMS and MARTIAN ANALOGS: David R
Lunar and Planetary Science XXXIV (2003) 1245.pdf COLD-BASED GLACIERS IN THE WESTERN DRY VALLEYS OF ANTARCTICA: TERRESTRIAL LANDFORMS AND MARTIAN ANALOGS: David R. Marchant1 and James W. Head2, 1Department of Earth Sciences, Boston University, Boston, MA 02215 [email protected], 2Department of Geological Sciences, Brown University, Providence, RI 02912 Introduction: Basal-ice and surface-ice temperatures are contacts and undisturbed underlying strata are hallmarks of cold- key parameters governing the style of glacial erosion and based glacier deposits [11]. deposition. Temperate glaciers contain basal ice at the pressure- Drop moraines: The term drop moraine is used here to melting point (wet-based) and commonly exhibit extensive areas describe debris ridges that form as supra- and englacial particles of surface melting. Such conditions foster basal plucking and are dropped passively at margins of cold-based glaciers (Fig. 1a abrasion, as well as deposition of thick matrix-supported drift and 1b). Commonly clast supported, the debris is angular and sheets, moraines, and glacio-fluvial outwash. Polar glaciers devoid of fine-grained sediment associated with glacial abrasion include those in which the basal ice remains below the pressure- [10, 12]. In the Dry Valleys, such moraines may be cored by melting point (cold-based) and, in extreme cases like those in glacier ice, owing to the insulating effect of the debris on the the western Dry Valleys region of Antarctica, lack surface underlying glacier. Where cored by ice, moraine crests can melting zones. These conditions inhibit significant glacial exceed the angle of repose. In plan view, drop moraines closely erosion and deposition. -

Avulsion Dynamics in a River with Alternating Bedrock and Alluvial Reaches, Huron River, Northern Ohio (USA)
Open Journal of Modern Hydrology, 2019, 9, 20-39 http://www.scirp.org/journal/ojmh ISSN Online: 2163-0496 ISSN Print: 2163-0461 Avulsion Dynamics in a River with Alternating Bedrock and Alluvial Reaches, Huron River, Northern Ohio (USA) Mark J. Potucek1,2, James E. Evans1 1Department of Geology, Bowling Green State University, Bowling Green, OH, USA 2Arizona Department of Water Resources, Phoenix, AZ, USA How to cite this paper: Potucek, M.J. and Abstract Evans, J.E. (2019) Avulsion Dynamics in a River with Alternating Bedrock and Alluvi- The Huron River consists of alternating bedrock reaches and alluvial reaches. al Reaches, Huron River, Northern Ohio Analysis of historical aerial photography from 1950-2015 reveals six major (USA). Open Journal of Modern Hydrolo- gy, 9, 20-39. channel avulsion events in the 8-km study area. These avulsions occurred in https://doi.org/10.4236/ojmh.2019.91002 the alluvial reaches but were strongly influenced by the properties of the up- stream bedrock reach (“inherited characteristics”). The bedrock reaches Received: December 19, 2018 Accepted: January 20, 2019 aligned with the azimuth of joint sets in the underlying bedrock. One inhe- Published: January 23, 2019 rited characteristic in the alluvial reach downstream is that the avulsion channels diverged only slightly from the orientation of the upstream bedrock Copyright © 2019 by author(s) and channel (range 2˚ - 38˚, mean and standard deviation 12.1˚ ± 13.7˚). A Scientific Research Publishing Inc. This work is licensed under the Creative second inherited characteristic is that avulsion channels were initiated from Commons Attribution International short distances downstream after exiting the upstream bedrock channel reach License (CC BY 4.0). -

Floods Floods Are the Most Common Natural Disasters in the Country
Floods Floods are the most common natural disasters in the country. However, not all floods are alike. Some can develop slowly over a long period of rain or during warm weather after a heavy snowfall. Others, such as flash floods, can happen quickly, even without any visible signs of rain. It is important to be prepared for flooding no matter where you live, but especially if you live in a low-lying area, near water or downstream from a dam. Even a very small stream or a dry creek bed can overflow and cause flooding. Prepare supplies Make a Plan Stay informed Prepare an emergency supply kit, Develop a family emergency plan. Know the terms which includes items like non- Your family may not be together in the perishable food, water, battery same place when disaster strikes, so Flash Flood Warning: there has been a sudden flood. Head for operated or crank radio, extra it is important to know how you will flashlights and batteries. Consider contact one another, how to get back higher ground immediately keeping a laptop computer in your together and what you will do in case height. vehicle. The kit should include: of emergency. Flood Warning: there has been Prescriptions Plan places where your family will a flood or will there be a flood Bottled water, a battery radio meet, both within and outside your soon. It is advised to evacuate and extra batteries, a first aid immediate neighborhood. immediately. kit and a flashlight. Copies of important Be sure to take into account the Flood Watch: It is possible that specific needs of family members. -

Sediment Discharge in the Upper Arroyo Grande and Santa Rita Creek Basins 23 5
PB 256 422 Sediment Discharge in the Upper Arroyo Grande and 41,111 anta Rita Creek Basins, tot,:San Luis Obispo County, 1144300,6k California U. S. GEOLOGICAL SURVPFY Water-Resources Investigations 151/4 76-64 101„,14.11kup lemw QE Prepared in cooperation with the 75 .U58w SAN LUIS OBISPO COUNTY no.76-64 1976 ENGINEERING DEPARTMENT lkigtikPHIC DATA I. ReportuNo.Gs 2. 3. Recipient's Accession No. S NRD/WRI-76/053 4. Title and Subtitle 5.keport Date WS14/3610WPOISCHARGE IN THE UPPER ARROYO GRANDE AND SANTA RITA June 1976,/ 6. ben,/ erctFardthf INS, SAN LUIS OBISPO COUNTY, CALIFORNIA (t;"f„ ,, ,/,,,,,,,, °./. Author(s) 8. Performing Organization Rept. .„ J. M. Knott,! N'- USGS/WRI 76-64 9. Performing Organization Name and Address 10. Project/Task/Work Unit No. U.SAeological Survey, Water Resources Division California District )- 11. Contract/Grant No. 345 Middlefield Road Menlo Park, California 94025 12. Sponsoring Organization Name and Address 13. Type of Report & Period U.S. Geological Survey, Water Resources Division Covered California District Final 345 Middlefield Road 14. Menlo Park, California 9409c 15. Supplementary Notes Prepared in cooperation wit igir. .ng Department 16.AbstractsSediment data collected in the upper Arroyo Grande and Santa Rita Creek basins during the 1968-73 water years were analyzed to determine total sediment discharge at four stations in the basins. Water discharge and total sediment discharge at these stations, representative of the 1943-72 period, were estimated from'long-term flow data for nearby gaging stations and water-sediment discharge relations determined for the 1968-73 water years. -

The Central Arroyo Stream Restoration Program
This presentation premiered at WaterSmart Innovations watersmartinnovations.com Breaking Down the Barriers to Generate Sustainable Water Solutions The Arroyo Seco – A Case Study Tim Brick and Eliza Jane Whitman Arroyo Seco Foundation The Arroyo Seco Major tributary of the Los Angeles River Linking downtown LA to the San Gabriel Mountains Home of JPL and the Rose Bowl Watershed Management Program for last ten years Corps Feasibility Study The Historic Arroyo Seco Two different views of the Colorado Street Bridge The Most Celebrated Canyon in Southern California “This arroyo would make one of the greatest parks in the world” - Theodore Roosevelt, 1911 Current Status of the Arroyo Seco Though long celebrated as one of the most beautiful streams in Southern California, the Arroyo Seco has not escaped wide-spread damage caused by human impact Damage Caused by Urbanization / Channelization * destruction of habitat and wildlife * reduced infiltration * impaired water quality Central Arroyo Planning How does a golf course fit into watershed management and restoration? Alternative Alignments for Stream Restoration Celebrating the Arroyo The Central Arroyo Stream Restoration Program Improving the health of a stream and ensuring the future and sustainability of Pasadena The Central Arroyo Brookside Golf Course Project Accomplishments Improved water quality Enhanced trail network Implemented runoff BMPs Restored aquatic habitat Reintroduced the native Arroyo Chub Provided a model of stream restoration for urban SoCal Improved Aquatic Habitat ☼ Created backwater pools and structures to provide resting, foraging, and spawning areas for fish. ☼ Stabilized stream banks ☼ Improvements were constructed with natural materials (native trees and arroyo Backwater pool and stone). woody debris ☼ Under direction of CDFG, reintroduced 300 arroyo chub to the central Arroyo Seco. -

The Effects of Longitudinal Differences in Gravel Mobility on the Downstream Fining Pattern in the Cosumnes River, California
The Effects of Longitudinal Differences in Gravel Mobility on the Downstream Fining Pattern in the Cosumnes River, California Candice R. Constantine,1 Jeffrey F. Mount, and Joan L. Florsheim Department of Geological Sciences, University of California, Davis, California 95616, U.S.A. (e-mail: [email protected]) ABSTRACT Downstream fining in the Cosumnes River is partially controlled by longitudinal variation in sediment mobility linked to changes in cross-sectional morphology. Strong fining occurs where the channel is self-formed with section- averaged bankfull dimensionless shear stress (t∗ ) near the threshold of motion (ca. 0.031), allowing for size-selective transport. In contrast, fining is minimal in confined reaches wheret∗ is generally greater than twice the threshold value and transport is nonselective. Downstream fining is best described by a model that depicts fining in discrete intervals separated by a segment in which equal mobility of bed material accounts for the lack of diminishing grain size. Introduction Reduction in the size of bed material with distance 1980; Rice and Church 1998; Rice 1999) or anthro- downstream is commonly observed in gravel-bed pogenic channel modifications (Surian 2002). rivers. Researchers have attributed this feature, The relative importance of sorting and abrasion termed “downstream fining,” to the processes of in determining the diminution coefficient depends selective sorting (Ashworth and Ferguson 1989; on the system in question, particularly on the na- Ferguson and Ashworth 1991; Paola et al. 1992; Fer- ture of material in transport (Bradley 1970; Parker guson et al. 1996; Seal et al. 1997) and abrasion 1991; Werrity 1992; Kodama 1994a, 1994b) and (Bradley 1970; Schumm and Stevens 1973; Werrity whether sediment discharge is limited by transport 1992; Kodama 1994a, 1994b).