Update on Tick-Borne Rickettsioses Around the World: a Geographic Approach
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Hplc-Uv Quantitation of Folate Synthesized by Rickettsia
HPLC-UV QUANTITATION OF FOLATE SYNTHESIZED BY RICKETTSIA ENDOSYMBIONT IXODES PACIFICUS (REIP) By Junyan Chen A Thesis Presented to The Faculty of Humboldt State University In Partial Fulfillment of the Requirements for the Degree Master of Science in Biology Committee Membership Dr. Jianmin Zhong, Committee Chair Dr. David S. Baston, Committee Member Dr. Jenny Cappuccio, Committee Member Dr. Jacob Varkey, Committee Member Dr. Erik Jules, Program Graduate Coordinator December 2017 ABSTRACT HPLC-UV QUANTITATION OF FOLATE SYNTHESIZED BY RICKETTSIA ENDOSYMBIONT IXODES PACIFICUS (REIP) Junyan Chen Ticks are the most important vector of many infectious diseases in the United States. Understanding the nature of the relationship between Rickettsia endosymbiont Ixodes pacificus (REIP) and Exudes pacificus will help develop strategies for the control of tick- borne diseases, such as Lyme disease, and Rocky Mountain spotted fever. Folate, also known as vitamin B9, is a necessary vitamin for tick survival, and plays a central role in one-carbon metabolism in cells. Folate exist as a large family of structurally related forms that transfer one-carbon groups among biomolecules that are important to cell growth, differentiation, and survival. In Dr. Zheng’s lab, REIP were cultured in Ixodes scapularis embryonic tick cell line ISE6. Previous research has shown that REIP in Ixodes pacificus carries all five de novo folate biosynthesis genes. Folate biosynthesis mRNAs were detected and all recombinant rickettsial folate proteins were overexpressed. To determine whether REIP synthesize folate, we sought to measure the folate concentration in REIP using HPLC-UV quantification with a Diamond HydrideTM liquid chromatography column. 5-methyltetrahydrofolate (5-MTHF), the active circulating form of folate in bacteria was detected. -

Comparative Population Genetic Structure of Two Ixodidae Ticks (Ixodes Ovatus and Haemaphysalis
bioRxiv preprint doi: https://doi.org/10.1101/862904; this version posted March 19, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. 1 Title 2 Comparative population genetic structure of two Ixodidae ticks (Ixodes ovatus and Haemaphysalis 3 flava) in Niigata Prefecture, Japan 4 Author names and affiliations 5 Maria Angenica Fulo Regilmea, Megumi Satob, Tsutomu Tamurac, Reiko Araic, Marcello Otake Satod, 6 Sumire Ikedae, Masaya Doia, Kohki Tanakaa, Maribet Gamboaa, Michael T. Monaghanf,g, Kozo 7 Watanabea, h 8 a Department of Civil and Environmental Engineering, Ehime University, Matsuyama, Ehime, 790- 9 8577, Japan 10 b Graduate School of Health Sciences, Niigata University, Niigata, 951-8518, Japan 11 c Niigata Prefectural Institute of Public Health and Environmental Sciences, Niigata, 950-2144, Japan 12 d Department of Tropical Medicine and Parasitology, Dokkyo Medical University, 880 Kitakobayashi, 13 Mibu-machi, Shimotsuga-gun, Tochigi 321-0293, Japan 14 e Research Laboratories, Research and Development Headquarters, Earth Corporation, Hyogo 678- 15 0192, Japan 16 f Leibniz-Institute of Freshwater Ecology and Inland Fisheries (IGB), Berlin, 12587, Germany 17 g Institut für Biologie, Freie Universität Berlin, 14195, Germany 18 h Center for Marine Environmental Studies (CMES), Ehime University, Matsuyama, Ehime, 790-8577, 19 Japan 20 Corresponding author 21 [email protected] 1 bioRxiv preprint doi: https://doi.org/10.1101/862904; this version posted March 19, 2020. -

Arthropod Parasites in Domestic Animals
ARTHROPOD PARASITES IN DOMESTIC ANIMALS Abbreviations KINGDOM PHYLUM CLASS ORDER CODE Metazoa Arthropoda Insecta Siphonaptera INS:Sip Mallophaga INS:Mal Anoplura INS:Ano Diptera INS:Dip Arachnida Ixodida ARA:Ixo Mesostigmata ARA:Mes Prostigmata ARA:Pro Astigmata ARA:Ast Crustacea Pentastomata CRU:Pen References Ashford, R.W. & Crewe, W. 2003. The parasites of Homo sapiens: an annotated checklist of the protozoa, helminths and arthropods for which we are home. Taylor & Francis. Taylor, M.A., Coop, R.L. & Wall, R.L. 2007. Veterinary Parasitology. 3rd edition, Blackwell Pub. HOST-PARASITE CHECKLIST Class: MAMMALIA [mammals] Subclass: EUTHERIA [placental mammals] Order: PRIMATES [prosimians and simians] Suborder: SIMIAE [monkeys, apes, man] Family: HOMINIDAE [man] Homo sapiens Linnaeus, 1758 [man] ARA:Ast Sarcoptes bovis, ectoparasite (‘milker’s itch’)(mange mite) ARA:Ast Sarcoptes equi, ectoparasite (‘cavalryman’s itch’)(mange mite) ARA:Ast Sarcoptes scabiei, skin (mange mite) ARA:Ixo Ixodes cornuatus, ectoparasite (scrub tick) ARA:Ixo Ixodes holocyclus, ectoparasite (scrub tick, paralysis tick) ARA:Ixo Ornithodoros gurneyi, ectoparasite (kangaroo tick) ARA:Pro Cheyletiella blakei, ectoparasite (mite) ARA:Pro Cheyletiella parasitivorax, ectoparasite (rabbit fur mite) ARA:Pro Demodex brevis, sebacceous glands (mange mite) ARA:Pro Demodex folliculorum, hair follicles (mange mite) ARA:Pro Trombicula sarcina, ectoparasite (black soil itch mite) INS:Ano Pediculus capitis, ectoparasite (head louse) INS:Ano Pediculus humanus, ectoparasite (body -

Diapause and Quiescence As Two Main Kinds of Dormancy and Their Significance in Life Cycles of Mites and Ticks (Chelicerata: Arachnida: Acari)
Acarina 17 (1): 3–32 © Acarina 2009 DIAPAUSE AND QUIESCENCE AS TWO MAIN KINDS OF DORMANCY AND THEIR SIGNIFICANCE IN LIFE CYCLES OF MITES AND TICKS (CHELICERATA: ARACHNIDA: ACARI). PART 2. PARASITIFORMES V. N. Belozerov Biological Research Institute, St. Petersburg State University, Peterhof 198504, Russia; e-mail: [email protected] ABSTRACT: Concerning the problem of life history and such an important its aspect as seasonality of life cycles and their control enabled by dormant stages, the parasitiform mites reveal the obvious similarity with the acariform mites. This concerns the pres- ence of both main kinds of dormancy (diapause and quiescence). The great importance in the seasonal control of life cycles in some parasitiform mites, like in acariform mites, belongs also for combinations of diapause with non-diapause arrests, particularly with the post-diapause quiescence (PDQ). This type of quiescence evoked after termination of diapause and enabling more accu- rate time-adjustment in recommencement of active development, is characteristic of both lineages of the Parasitiformes — Ixodida and Mesostigmata (particularly Gamasida). The available data show that in ixodid ticks the PDQ may be resulted similarly after developmental and behavioral diapause. Reproductive diapause combined with the PDQ is characteristic of some gamasid mites (particularly the family Phytoseiidae), while most gamasid and uropodid mites with phoretic dispersal reveal the dormant state (apparently of diapause nature) at the deutonymphal stage. The uncertainty between diapause and non-diapause dormancy is retained in some many cases (even in ixodid ticks and phytoseiid mites), and the necessity of further thorough study of different forms of diapause and non-diapause arrests in representatives of the Acari is noted therefore. -

Annotated Bibliography for Barrow Island Terrestrial Invertebrates
RECORDS OF THE WESTERN AUSTRALIAN MUSEUM 83 135–144 (2013) DOI: 10.18195/issn.0313-122x.83.2013.135-144 SUPPLEMENT Annotated bibliography for Barrow Island terrestrial invertebrates Christopher K. Taylor Department of Environment and Agriculture, Curtin University, GPO Box U1987, Perth, Western Australia 6845, Australia. Email: [email protected] ABSTRACT – A bibliography is provided of publications treating terrestrial invertebrates on Barrow Island. A brief overview is also given of natural history and invertebrate collections on Barrow Island. KEYWORDS: Arthropoda, Insecta, Arachnida, Gastropoda, publication history INTRODUCTION During the late 1800s Barrow Island was utilised at various times by pastoralists, guano miners, pearl As part of this special issue on the terrestrial and turtle fishers, and slavers (Hook et al. 2004; invertebrate fauna of Barrow Island in Western ‘Supreme Court—Civil Side’, West Australian, 26 Australia, we take the opportunity to present May 1887; ‘The native question’, Daily News [Perth], a bibliography of previous publications on the 16 February 1905). If any of these individuals subject. A more general bibliography of Barrow were interested in collecting invertebrates, their Island’s natural history was previously collated by endeavours in that field have not been recorded for Smith et al. (2006). The current bibliography differs posterity. from that in gathering not only publications for which Barrow Island was the primary focus, but J.T. Tunney of the Western Australian Museum also those in which Barrow Island specimens were spent six weeks on Barrow Island in 1901 (‘News considered as part of a broader study. and notes’, West Australian, 22 March 1901). -

Morphological and Molecular Description of Ixodes Woyliei N. Sp
Ash et al. Parasites & Vectors (2017) 10:70 DOI 10.1186/s13071-017-1997-8 RESEARCH Open Access Morphological and molecular description of Ixodes woyliei n. sp. (Ixodidae) with consideration for co-extinction with its critically endangered marsupial host Amanda Ash1*, Aileen Elliot1, Stephanie Godfrey1, Halina Burmej1, Mohammad Yazid Abdad1,2, Amy Northover1, Adrian Wayne3, Keith Morris4, Peta Clode5, Alan Lymbery1 and R. C. Andrew Thompson1 Abstract Background: Taxonomic identification of ticks obtained during a longitudinal survey of the critically endangered marsupial, Bettongia penicillata Gray, 1837 (woylie, brush-tailed bettong) revealed a new species of Ixodes Latrielle, 1795. Here we provide morphological data for the female and nymphal life stages of this novel species (Ixodes woyliei n. sp.), in combination with molecular characterisation using the mitochondrial cytochrome c oxidase subunit 1 gene (cox1). In addition, molecular characterisation was conducted on several described Ixodes species and used to provide phylogenetic context. Results: Ixodes spp. ticks were collected from the two remaining indigenous B. penicillata populations in south- western Australia. Of 624 individual B. penicillata sampled, 290 (47%) were host to ticks of the genus Ixodes; specifically I. woyliei n. sp., I. australiensis Neumann, 1904, I. myrmecobii Roberts, 1962, I. tasmani Neumann, 1899 and I. fecialis Warburton & Nuttall, 1909. Of these, 123 (42%) were host to the newly described I. woyliei n. sp. In addition, 268 individuals from sympatric marsupial species (166 Trichosurus vulpecula hypoleucus Wagner, 1855 (brushtail possum), 89 Dasyurus geoffroii Gould, 1841 (Western quoll) and 13 Isoodon obesulus fusciventer Gray, 1841 (southern brown bandicoot)) were sampled for ectoparasites and of these, I. -

Ehrlichia, and Anaplasma Species in Australian Human-Biting Ticks
RESEARCH ARTICLE Bacterial Profiling Reveals Novel “Ca. Neoehrlichia”, Ehrlichia, and Anaplasma Species in Australian Human-Biting Ticks Alexander W. Gofton1*, Stephen Doggett2, Andrew Ratchford3, Charlotte L. Oskam1, Andrea Paparini1, Una Ryan1, Peter Irwin1* 1 Vector and Water-borne Pathogen Research Group, School of Veterinary and Life Sciences, Murdoch University, Perth, Western Australia, Australia, 2 Department of Medical Entomology, Pathology West and Institute for Clinical Pathology and Medical Research, Westmead Hospital, Westmead, New South Wales, Australia, 3 Emergency Department, Mona Vale Hospital, New South Wales, Australia * [email protected] (AWG); [email protected] (PI) Abstract OPEN ACCESS In Australia, a conclusive aetiology of Lyme disease-like illness in human patients remains Citation: Gofton AW, Doggett S, Ratchford A, Oskam elusive, despite growing numbers of people presenting with symptoms attributed to tick CL, Paparini A, Ryan U, et al. (2015) Bacterial bites. In the present study, we surveyed the microbial communities harboured by human-bit- Profiling Reveals Novel “Ca. Neoehrlichia”, Ehrlichia, ing ticks from across Australia to identify bacteria that may contribute to this syndrome. and Anaplasma Species in Australian Human-Biting Ticks. PLoS ONE 10(12): e0145449. doi:10.1371/ Universal PCR primers were used to amplify the V1-2 hyper-variable region of bacterial journal.pone.0145449 16S rRNA genes in DNA samples from individual Ixodes holocyclus (n = 279), Amblyomma Editor: Bradley S. Schneider, Metabiota, UNITED triguttatum (n = 167), Haemaphysalis bancrofti (n = 7), and H. longicornis (n = 7) ticks. STATES The 16S amplicons were sequenced on the Illumina MiSeq platform and analysed in Received: October 12, 2015 USEARCH, QIIME, and BLAST to assign genus and species-level taxonomies. -
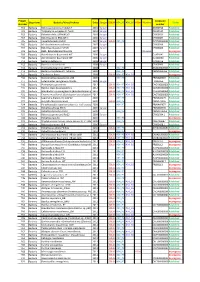
Project Number Organisms Bacteria/Virus/Archaea Date
Project_ Accession Organisms Bacteria/Virus/Archaea Date Sanger SOLiD 454_PE 454_SG PGM Illumina Status Number number P01 Bacteria Rickettsia conorii str.Malish 7 2001 Sanger AE006914 Published P02 Bacteria Tropheryma whipplei str.Twist 2003 Sanger AE014184 Published P03 Bacteria Rickettsia felis URRWXCal2 2005 Sanger CP000053 Published P04 Bacteria Rickettsia bellii RML369-C 2006 Sanger CP000087 Published P05 Bacteria Coxiella burnetii CB109 2007 Sanger SOLiD 454_PE AKYP00000000 Published P06 Bacteria Minibacterium massiliensis 2007 Sanger CP000269 Published P07 Bacteria Rickettsia massiliae MTU5 2007 Sanger CP000683 Published P08 Bacteria BaBL=Bête à Bernard Lascola 2007 Illumina In progress P09 Bacteria Acinetobacter baumannii AYE 2006 Sanger CU459141 Published P10 Bacteria Acinetobacter baumannii SDF 2006 Sanger CU468230 Published P11 Bacteria Borrelia duttonii Ly 2008 Sanger CP000976 Published P12 Bacteria Borrelia recurrentis A1 2008 Sanger CP000993 Published P13 Bacteria Francisella tularensis URFT1 2008 454_PE ABAZ00000000Published P14 Bacteria Borrelia crocidurae str. Achema 2009 454_PE PRJNA162335 Published P15 Bacteria Citrobacter koseri 2009 SOLiD 454_PE 454_SG In progress P16 Bacteria Diplorickettsia massiliensis 20B 2009 454_PE PRJNA86907 Published P17 Bacteria Enterobacter aerogenes EA1509E 2009 Sanger FO203355 Published P18 Bacteria Actinomyces grossensis 2012 SOLiD 454_PE 454_SG CAGY00000000Published P19 Bacteria Bacillus massiliosenegalensis 2012 SOLiD 454_PE 454_SG CAHJ00000000 Published P20 Bacteria Brevibacterium senegalensis -

Scoping Review of Distribution Models for Selected Amblyomma Ticks and Rickettsial Group Pathogens
Scoping review of distribution models for selected Amblyomma ticks and rickettsial group pathogens Catherine A. Lippi1,2, Holly D. Gaff3,4, Alexis L. White1,2 and Sadie J. Ryan1,2 1 Department of Geography, University of Florida, Gainesville, FL, USA 2 Emerging Pathogens Institute, University of Florida, Gainesville, FL, USA 3 Department of Biology, Old Dominion University, Norfolk, VA, USA 4 School of Mathematics, Statistics and Computer Science, University of Kwa-Zulu Natal, Durban, South Africa ABSTRACT The rising prevalence of tick-borne diseases in humans in recent decades has called attention to the need for more information on geographic risk for public health planning. Species distribution models (SDMs) are an increasingly utilized method of constructing potential geographic ranges. There are many knowledge gaps in our understanding of risk of exposure to tick-borne pathogens, particularly for those in the rickettsial group. Here, we conducted a systematic scoping review of the SDM literature for rickettsial pathogens and tick vectors in the genus Amblyomma. Of the 174 reviewed articles, only 24 studies used SDMs to estimate the potential extent of vector and/or pathogen ranges. The majority of studies (79%) estimated only tick distributions using vector presence as a proxy for pathogen exposure. Studies were conducted at different scales and across multiple continents. Few studies undertook original data collection, and SDMs were mostly built with presence-only datasets from public database or surveillance sources. The reliance on existing data sources, using ticks as a proxy for disease risk, may simply reflect a lag in new data acquisition and a thorough understanding of the tick-pathogen ecology involved. -

Diversity of Spotted Fever Group Rickettsiae and Their Association
www.nature.com/scientificreports OPEN Diversity of spotted fever group rickettsiae and their association with host ticks in Japan Received: 31 July 2018 May June Thu1,2, Yongjin Qiu3, Keita Matsuno 4,5, Masahiro Kajihara6, Akina Mori-Kajihara6, Accepted: 14 December 2018 Ryosuke Omori7,8, Naota Monma9, Kazuki Chiba10, Junji Seto11, Mutsuyo Gokuden12, Published: xx xx xxxx Masako Andoh13, Hideo Oosako14, Ken Katakura2, Ayato Takada5,6, Chihiro Sugimoto5,15, Norikazu Isoda1,5 & Ryo Nakao2 Spotted fever group (SFG) rickettsiae are obligate intracellular Gram-negative bacteria mainly associated with ticks. In Japan, several hundred cases of Japanese spotted fever, caused by Rickettsia japonica, are reported annually. Other Rickettsia species are also known to exist in ixodid ticks; however, their phylogenetic position and pathogenic potential are poorly understood. We conducted a nationwide cross-sectional survey on questing ticks to understand the overall diversity of SFG rickettsiae in Japan. Out of 2,189 individuals (19 tick species in 4 genera), 373 (17.0%) samples were positive for Rickettsia spp. as ascertained by real-time PCR amplifcation of the citrate synthase gene (gltA). Conventional PCR and sequencing analyses of gltA indicated the presence of 15 diferent genotypes of SFG rickettsiae. Based on the analysis of fve additional genes, we characterised fve Rickettsia species; R. asiatica, R. helvetica, R. monacensis (formerly reported as Rickettsia sp. In56 in Japan), R. tamurae, and Candidatus R. tarasevichiae and several unclassifed SFG rickettsiae. We also found a strong association between rickettsial genotypes and their host tick species, while there was little association between rickettsial genotypes and their geographical origins. -
Widespread Use of Realtime PCR for Rickettsial Diagnosis
SHORT COMMUNICATION Widespread use of real-time PCR for rickettsial diagnosis Aure´ lie Renvoise´ , Jean-Marc Rolain, Cristina Socolovschi & Didier Raoult Unite´ de Recherche en Maladies Infectieuses et Tropicales Emergentes CNRS-IRD UMR6236-198, Faculte´ de Me´ decine, Universite´ de la Me´ diterrane´ e, Marseille, France Correspondence: Didier Raoult, Unite´ de Abstract Recherche en Maladies Infectieuses et Tropicales Emergentes CNRS-IRD UMR6236- We report 2 years of experience with rickettsial molecular diagnosis using real- 198, Universite´ de la Me´ diterrane´ e, Faculte´ time PCR at the French National Reference Center. All Rickettsia genomes avail- de Me´ decine, 27 bd Jean Moulin, 13385 able were compared to discover specific sequences to design new sets of primers Marseille Cedex 5, France. Tel.: and probes. The specificity was verified in silico and against a panel of 30 rick- +33491324375; fax: +33491387772; ettsial species. Sensitivity was determined using 10-fold serial dilutions. Finally, e-mail: [email protected] primers and probes that were both specific and sensitive were routinely used Received 6 July 2011; revised 6 October for the diagnosis of rickettsial infections from clinical specimens. We retained 2011; accepted 31 October 2011. sets of primers and probes to detect spotted fever group Rickettsia, typhus Final version published online 8 December group Rickettsia, Rickettsia conorii, Rickettsia slovaca, Rickettsia africae and Rick- 2011. ettsia australis; 643 clinical samples were screened for the presence of Rickettsia DNA. Overall, 45 positive samples were detected, including 15 Rickettsia africae, DOI: 10.1111/j.1574-695X.2011.00899.x nine R. conorii, five Rickettsia sibirica mongolitimonae, four R. -

Bacterial Diversity in Amblyomma Americanum (Acari: Ixodidae) with Afocusonmembersofthegenusrickettsia
VECTOR-BORNE DISEASES,SURVEILLANCE,PREVENTION Bacterial Diversity in Amblyomma americanum (Acari: Ixodidae) With aFocusonMembersoftheGenusRickettsia 1 2 1 STEPHANIE R. HEISE, M. S. ELSHAHED, AND S. E. LITTLE Department of Veterinary Pathobiology, Center for Veterinary Health Sciences, Oklahoma State University, Stillwater, OK 74078 J. Med. Entomol. 47(2): 258Ð268 (2010); DOI: 10.1603/ME09197 ABSTRACT The lone star tick, Amblyomma americanum (Acari: Ixodidae), is commonly reported from people and animals throughout the eastern U.S. and is associated with transmission of a number of emerging diseases. To better deÞne the microbial communities within lone star ticks, 16S rRNA gene based analysis using bacteria-wide primers, followed by sequencing of individual clones (n ϭ 449) was used to identify the most common bacterial operational taxonomic units (OTUs) present within colony-reared and wild A. americanum.Thecolony-rearedtickscontainedprimarilysequenceafÞl- iated with members of the genus Coxiella (89%; 81/91), common endosymbionts of ticks, and Brevibacterium (11%; 10/91). Similarly, analysis of clones from unfed wild lone star ticks revealed that 96.7% (89/92) of all the OTUs identiÞed were afÞliated with Coxiella-like endosymbionts, as compared with only 5.1Ð11.7% (5/98Ð9/77) of those identiÞed from wild lone star ticks after feeding. In contrast, the proportion of OTUs identiÞed as Rickettsia sp. in wild-caught ticks increased from 2.2% (2/92) before feeding to as high as 46.8% (36/77) after feeding, and all Rickettsia spp. sequences recovered were most similar to those described from the spotted fever group Rickettsia,speciÞcallyR. amblyo- mmii and R. massiliae.AdditionalcharacterizationoftheRickettsialestickcommunitybypolymerase chain reaction, cloning, and sequencing of 17 kDa and gltA genes conÞrmed these initial Þndings and suggested that novel Rickettsia spp.