0881NC Revised.Pub
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

RESTORATION ACTION PLAN MARINA DUNES PRESERVE Marina, California
RESTORATION ACTION PLAN MARINA DUNES PRESERVE Marina, California Prepared for: Monterey Peninsula Regional Park District 4860 Carmel Valley Road Carmel, CA 93923 Prepared by: Burleson Consulting Inc. 1900 Garden Road, Suite 210 Monterey, CA 93940 March 2021 This page intentionally left blank Restoration Action Plan, Marina Dunes Preserve CONTENTS CONTENTS ..........................................................................................................................................i APPENDICES ...................................................................................................................................... ii ACRONYMS AND ABBREVIATIONS ..................................................................................................... iii 1. INTRODUCTION ...................................................................................................................... 1 1.1 Setting ........................................................................................................................................... 1 1.2 Purpose ......................................................................................................................................... 1 1.3 Approach ....................................................................................................................................... 2 2. UPDATED BEST MANAGEMENT PRACTICES .............................................................................. 3 2.1 Weed Eradication and Control ..................................................................................................... -
Plant Field Guide
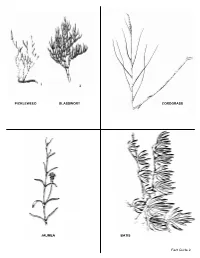
2 PICKLEWEED GLASSWORT CORDGRASS JAUMEA BATIS Field Guide 9 PICKLEWEED Amaranth Family 3 kinds, 2 examples CORDGRASS Grass Family 1 Pickleweed Sarcocornia pacifica Spartina foliosa Glasswort Arthrocnemum subterminalis 2 HABITAT: Growns in the low marsh where the HABITAT: Found throughout the salt marsh. roots are continually bathed in ocean water. APPEARANCE: Stems look like a chain of small APPEARANCE: Look for a tall grass which is pickles. higher than the other plants in the salt marsh. REPRODUCTION: The flowers of all pickleweeds REPRODUCTION: All grasses are wind pollinated. are pollinated by the wind. The small flowers are Look for straw colored spikes of densely packed hard to see because they have no colorful petals flowers. Male flowers will have pollen and the female flowers will show graceful waving stigmas to ADAPTATION TO SALT: Pickleweeds are some of catch the pollen. the many marsh plants that use salt storage (they are accumulators). Also called succulents, these ADAPTATION TO SALT: All the salt marsh plants are swollen with the stored salty water. grasses are salt excreters using special pores to When the salt concentration becomes too high the push out droplets of salty water. Look on the grass cells will die. blades for salt crystals. See sea lavender. ECOLOGICAL RELATIONSHIPS: Frequently the ECOLOGICAL RELATIONSHIPS: Home for the most common plants in the marsh, they provide endangered bird, the Light-footed Clapper Rail. shelter and food for invertebrates. Belding’s A spider lives its whole life inside the blades. Savannah Sparrows build their nests in the Important food for grazing animals. glasswort. BATIS or SALTWORT Saltwort Family Batis maritima HABITAT: Most frequently found in the low marsh. -

Iconic Bees: 12 Reports on UK Bee Species
Iconic Bees: 12 reports on UK bee species Bees are vital to the ecology of the UK and provide significant social and economic benefits through crop pollination and maintaining the character of the landscape. Recent years have seen substantial declines in many species of bees within the UK. This report takes a closer look at how 12 ‘iconic’ bee species are faring in each English region, as well as Wales, Northern Ireland and Scotland. Authors Rebecca L. Evans and Simon G. Potts, University of Reading. Photo: © Amelia Collins Contents 1 Summary 2 East England Sea-aster Mining Bee 6 East Midlands Large Garden Bumblebee 10 London Buff-tailed Bumblebee 14 North East Bilberry Bumblebee 18 North West Wall Mason Bee 22 Northern Ireland Northern Colletes 26 Scotland Great Yellow Bumblebee 30 South East England Potter Flower Bee 34 South West England Scabious Bee 38 Wales Large Mason Bee 42 West Midlands Long-horned Bee 46 Yorkshire Tormentil Mining Bee Through collating information on the 12 iconic bee species, common themes have Summary emerged on the causes of decline, and the actions that can be taken to help reverse it. The most pervasive causes of bee species decline are to be found in the way our countryside has changed in the past 60 years. Intensification of grazing regimes, an increase in pesticide use, loss of biodiverse field margins and hedgerows, the trend towards sterile monoculture, insensitive development and the sprawl of towns and cities are the main factors in this. I agree with the need for a comprehensive Bee Action Plan led by the UK Government in order to counteract these causes of decline, as called for by Friends of the Earth. -

Brassicaceae) in Australia
Cunninghamia Date of Publication: 26/08/2013 A journal of plant ecology for eastern Australia ISSN 0727- 9620 (print) • ISSN 2200 - 405X (Online) Reassessment of the invasion history of two species of Cakile (Brassicaceae) in Australia Roger D. Cousens, Peter K. Ades, Mohsen B. Mesgaran and Sara Ohadi Melbourne School of Land & Environment, The University of Melbourne, Victoria, 3010, AUSTRALIA Abstract: In this paper we revisit the invasion history of two species of Cakile in Australia. Cakile edentula subsp. edentula arrived in the mid 19th Century and spread into coastal strandline habitat from the southeast towards the west and to the north; Cakile maritima arrived in the late 19th Century and has replaced Cakile edentula over much of the range. While Cakile edentula is morphologically quite uniform, the great variation within Cakile maritima has confused field ecologists. Using herbarium records we update previous accounts of the spread of the species and report on field surveys that determined their current geographic overlap in Tasmania and in northern New South Wales/southern Queensland. We examine regional morphological variation within Cakile maritima using the national herbaria collections and variation within new population samples. We support previous interpretations that Cakile maritima has been introduced on more than one occasion from morphologically distinct races, resulting in regional variation within Australia and high variability within populations in the south-east. Western Australian populations appear distinct and probably did not initiate those in the east; we consider that eastern populations are likely to be a mix of Cakile maritima subsp. maritima from the Mediterranean and Cakile maritima subsp. -

State of New York City's Plants 2018
STATE OF NEW YORK CITY’S PLANTS 2018 Daniel Atha & Brian Boom © 2018 The New York Botanical Garden All rights reserved ISBN 978-0-89327-955-4 Center for Conservation Strategy The New York Botanical Garden 2900 Southern Boulevard Bronx, NY 10458 All photos NYBG staff Citation: Atha, D. and B. Boom. 2018. State of New York City’s Plants 2018. Center for Conservation Strategy. The New York Botanical Garden, Bronx, NY. 132 pp. STATE OF NEW YORK CITY’S PLANTS 2018 4 EXECUTIVE SUMMARY 6 INTRODUCTION 10 DOCUMENTING THE CITY’S PLANTS 10 The Flora of New York City 11 Rare Species 14 Focus on Specific Area 16 Botanical Spectacle: Summer Snow 18 CITIZEN SCIENCE 20 THREATS TO THE CITY’S PLANTS 24 NEW YORK STATE PROHIBITED AND REGULATED INVASIVE SPECIES FOUND IN NEW YORK CITY 26 LOOKING AHEAD 27 CONTRIBUTORS AND ACKNOWLEGMENTS 30 LITERATURE CITED 31 APPENDIX Checklist of the Spontaneous Vascular Plants of New York City 32 Ferns and Fern Allies 35 Gymnosperms 36 Nymphaeales and Magnoliids 37 Monocots 67 Dicots 3 EXECUTIVE SUMMARY This report, State of New York City’s Plants 2018, is the first rankings of rare, threatened, endangered, and extinct species of what is envisioned by the Center for Conservation Strategy known from New York City, and based on this compilation of The New York Botanical Garden as annual updates thirteen percent of the City’s flora is imperiled or extinct in New summarizing the status of the spontaneous plant species of the York City. five boroughs of New York City. This year’s report deals with the City’s vascular plants (ferns and fern allies, gymnosperms, We have begun the process of assessing conservation status and flowering plants), but in the future it is planned to phase in at the local level for all species. -

Mating Cluster Behaviour in the Solitary Bee Colletes Succinctus
To confirm species identification a small sample was Mating cluster behaviour in the collected in 2006 when the population reappeared. The identity was established from a male specimen whose solitary bee Colletes succinctus morphological characters, including features in the (Linn.), Hymenoptera, Colletidae genitalia, match those of Colletes succinctus , the girdled Colletes (Saunders, 1896; Step, 1946). This is 1 1 a common species on heaths and commons and usually Pauline Lang , E. Geoffrey Hancock , Kevin forages on heather. 1 2 J. Murphy & Robert L. Watt 1Division of Environmental Biology and Evolutionary Biology, University of Glasgow, Glasgow, Scotland G12 8QQ 2Bogendreip Farm, Strachan, Banchory, Kincardineshire, Scotland AB31 6LP In August 2005, during a botanical freshwater survey of the Water of Dye, a tributary of the River Dee at the Bridge of Bogendreip, Kincardineshire (NGR: NO 662910), our attention was drawn to the behaviour of a population of solitary bees. These bees were occupying plots of soil in the garden of Bogendreip Farm (Fig 1). Only in the last few years had Mr. Watt Fig. 2. Mating cluster in Colletes succinctus. observed the bees and not previously, in his more than fifty years of farm occupancy. The bees were A related species, Colletes hederae (Schmidt & subsequently identified as Colletes succinctus (Linn.). Westrich), the ivy bee, has been observed to form small knots of males when competing for females. A The bees had colonized extensive areas of sandy soils coloured photograph of a small group is shown in adjacent to the farmhouse, in which hundreds of Moenen (2005). Other species of the genus also individual nests formed two main “villages” with exhibit mating clusters. -

Wild Bees in the Hoeksche Waard
Wild bees in the Hoeksche Waard Wilson Westdijk C.S.G. Willem van Oranje Text: Wilson Westdijk Applicant: C.S.G. Willem van Oranje Contact person applicant: Bart Lubbers Photos front page Upper: Typical landscape of the Hoeksche Waard - Rotary Hoeksche Waard Down left: Andrena rosae - Gert Huijzers Down right: Bombus muscorum - Gert Huijzers Table of contents Summary 3 Preface 3 Introduction 4 Research question 4 Hypothesis 4 Method 5 Field study 5 Literature study 5 Bee studies in the Hoeksche Waard 9 Habitats in the Hoeksche Waard 11 Origin of the Hoeksche Waard 11 Landscape and bees 12 Bees in the Hoeksche Waard 17 Recorded bee species in the Hoeksche Waard 17 Possible species in the Hoeksche Waard 22 Comparison 99 Compared to Land van Wijk en Wouden 100 Species of priority 101 Species of priority in the Hoeksche Waard 102 Threats 106 Recommendations 108 Conclusion 109 Discussion 109 Literature 111 Sources photos 112 Attachment 1: Logbook 112 2 Summary At this moment 98 bee species have been recorded in the Hoeksche Waard. 14 of these species are on the red list. 39 species, that have not been recorded yet, are likely to occur in the Hoeksche Waard. This results in 137 species, which is 41% of all species that occur in the Netherlands. The species of priority are: Andrena rosae, A. labialis, A. wilkella, Bombus jonellus, B. muscorum and B. veteranus. Potential species of priority are: Andrena pilipes, A. gravida Bombus ruderarius B. rupestris and Nomada bifasciata. Threats to bees are: scaling up in agriculture, eutrophication, reduction of flowers, pesticides and competition with honey bees. -

Scottish Bees
Scottish Bees Introduction to bees Bees are fascinating insects that can be found in a broad range of habitats from urban gardens to grasslands and wetlands. There are over 270 species of bee in the UK in 6 families - 115 of these have been recorded in Scotland, with 4 species now thought to be extinct and insufficient data available for another 2 species. Bees are very diverse, varying in size, tongue-length and flower preference. In the UK we have 1 species of honey bee, 24 species of bumblebee and the rest are solitary bees. They fulfil an essential ecological and environmental role as one of the most significant groups of pollinating insects, all of which we depend upon for the pollination of 80% of our wild and cultivated plants. Some flowers are in fact designed specifically for bee pollination, to the exclusion of generalist pollinators. Bees and their relatives Bees are classified in the complex insect order Hymenoptera (meaning membrane-winged), which also includes many kinds of parasitic wasps, gall wasps, hunting wasps, ants and sawflies. There are about 150,000 species of Hymenoptera known worldwide separated into two sub-orders. The first is the most primitive sub-order Symphyta which includes the sawflies and their relatives, lacking a wasp-waist and generally with free-living caterpillar-like larvae. The second is the sub-order Apocrita, which includes the ants, bees and wasps which are ’wasp-waisted’ and have grub-like larvae that develop within hosts, galls or nests. The sub-order Apocrita is in turn divided into two sections, the Parasitica and Aculeata. -

Fort Ord Natural Reserve Plant List
UCSC Fort Ord Natural Reserve Plants Below is the most recently updated plant list for UCSC Fort Ord Natural Reserve. * non-native taxon ? presence in question Listed Species Information: CNPS Listed - as designated by the California Rare Plant Ranks (formerly known as CNPS Lists). More information at http://www.cnps.org/cnps/rareplants/ranking.php Cal IPC Listed - an inventory that categorizes exotic and invasive plants as High, Moderate, or Limited, reflecting the level of each species' negative ecological impact in California. More information at http://www.cal-ipc.org More information about Federal and State threatened and endangered species listings can be found at https://www.fws.gov/endangered/ (US) and http://www.dfg.ca.gov/wildlife/nongame/ t_e_spp/ (CA). FAMILY NAME SCIENTIFIC NAME COMMON NAME LISTED Ferns AZOLLACEAE - Mosquito Fern American water fern, mosquito fern, Family Azolla filiculoides ? Mosquito fern, Pacific mosquitofern DENNSTAEDTIACEAE - Bracken Hairy brackenfern, Western bracken Family Pteridium aquilinum var. pubescens fern DRYOPTERIDACEAE - Shield or California wood fern, Coastal wood wood fern family Dryopteris arguta fern, Shield fern Common horsetail rush, Common horsetail, field horsetail, Field EQUISETACEAE - Horsetail Family Equisetum arvense horsetail Equisetum telmateia ssp. braunii Giant horse tail, Giant horsetail Pentagramma triangularis ssp. PTERIDACEAE - Brake Family triangularis Gold back fern Gymnosperms CUPRESSACEAE - Cypress Family Hesperocyparis macrocarpa Monterey cypress CNPS - 1B.2, Cal IPC -

Mesgaran, M. B., Lewis, M. A., Ades, P. K., Donohue, K
Hybridization can facilitate species invasions, even without enhancing local adaptation Mohsen B. Mesgarana, Mark A. Lewisb, Peter K. Adesc, Kathleen Donohued, Sara Ohadia, Chengjun Lia, and Roger D. Cousensa,1 aSchool of BioSciences, The University of Melbourne, Victoria 3010, Australia; bMathematical and Statistical Sciences, University of Alberta, Edmonton, AB, Canada T6G 2G1; cSchool of Ecosystem and Forest Sciences, The University of Melbourne, Victoria 3010, Australia; and dDepartment of Biology, Duke University, Durham, NC 27708 Edited by Alan Hastings, University of California, Davis, CA, and approved July 5, 2016 (received for review April 6, 2016) The founding population in most new species introductions, or at the low pollinator visitation, or both (8, 9); the term “pollen limitation” leading edge of an ongoing invasion, is likely to be small. Severe isoftenusedasagenerictermwhen the exact mechanism is un- Allee effects—reductions in individual fitness at low population den- clear. In the extreme case of a single arriving adult, population sity—may then result in a failure of the species to colonize, even if persistence would normally be impossible unless the species is ca- the habitat could support a much larger population. Using a simula- pable of asexual reproduction or self-fertilization [“Baker’sLaw” tion model for plant populations that incorporates demography, mat- (10)]. Here, we propose a positive role for hybridization in species ing systems, quantitative genetics, and pollinators, we show that invasions and range expansion, a purely demographic mechanism Allee effects can potentially be overcome by transient hybridization without the requirement for any new adaptation to result. with a resident species or an earlier colonizer. -

Rvk-Diss Digi
University of Groningen Of dwarves and giants van Klink, Roel IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's PDF) if you wish to cite from it. Please check the document version below. Document Version Publisher's PDF, also known as Version of record Publication date: 2014 Link to publication in University of Groningen/UMCG research database Citation for published version (APA): van Klink, R. (2014). Of dwarves and giants: How large herbivores shape arthropod communities on salt marshes. s.n. Copyright Other than for strictly personal use, it is not permitted to download or to forward/distribute the text or part of it without the consent of the author(s) and/or copyright holder(s), unless the work is under an open content license (like Creative Commons). The publication may also be distributed here under the terms of Article 25fa of the Dutch Copyright Act, indicated by the “Taverne” license. More information can be found on the University of Groningen website: https://www.rug.nl/library/open-access/self-archiving-pure/taverne- amendment. Take-down policy If you believe that this document breaches copyright please contact us providing details, and we will remove access to the work immediately and investigate your claim. Downloaded from the University of Groningen/UMCG research database (Pure): http://www.rug.nl/research/portal. For technical reasons the number of authors shown on this cover page is limited to 10 maximum. Download date: 01-10-2021 Of Dwarves and Giants How large herbivores shape arthropod communities on salt marshes Roel van Klink This PhD-project was carried out at the Community and Conservation Ecology group, which is part of the Centre for Ecological and Environmental Studies of the University of Groningen, The Netherlands. -

Characterization of Mycotoxigenic Alternaria Species Isolated from the Tunisian Halophyte Citation: A
Phytopathologia Mediterranea Firenze University Press The international journal of the www.fupress.com/pm Mediterranean Phytopathological Union Research Paper Characterization of mycotoxigenic Alternaria species isolated from the Tunisian halophyte Citation: A. Chalbi, B. Sghaier-Ham- mami, G. Meca, J.M. Quiles, C. Abdel- Cakile maritima ly, C. Marangi, A.F. Logrieco, A. Moret- ti, M. Masiello (2020) Characterization of mycotoxigenic Alternaria species isolated from the Tunisian halophyte Arbia CHALBI1,2,3, Besma SGHAIER-HAMMAMI1,*, Giuseppe MECA4, Cakile maritima. Phytopathologia Medi- Juan Manuel QUILES4, Chedly ABDELLY1, Carmela MARANGI5, Anto- terranea 59(1): 107-118. doi: 10.14601/ nio F. LOGRIECO3, Antonio MORETTI3, Mario MASIELLO3 Phyto-10720 1 Laboratoire des Plantes Extrêmophiles, Centre de Biotechnologie de Borj-Cédria, BP Accepted: February 7, 2020 901, Hammam-Lif 2050, Tunisia 2 Published: April 30, 2020 Faculty of Sciences of Tunis, University Campus 2092, University of Tunis El Manar, Tunis, Tunisia Copyright: © 2020 A. Chalbi, B. 3 Institute of Sciences of Food Production, Research National Council (ISPA-CNR), Via G. Sghaier-Hammami, G. Meca, J.M. Amendola 122/O, 70126, Bari, Italy Quiles, C. Abdelly, C. Marangi, A.F. 4 Laboratory of Food Toxicology, Department of Preventive Medicine, Nutrition and Food Logrieco, A. Moretti, M. Masiello. This Science Area, Faculty of Pharmacy, University of Valencia Avenida Vicent Andres Estelles is an open access, peer-reviewed s/n, 46100 Burjassot, Valencia, Spain article published by Firenze Univer- 5 sity Press (http://www.fupress.com/pm) Institute for Applied Mathematics “M. Picone”, Via G. Amendola 122/D, 70126, Bari, and distributed under the terms of the Italy Creative Commons Attribution License, * Corresponding author: [email protected] which permits unrestricted use, distri- bution, and reproduction in any medi- um, provided the original author and Summary.