Carcinoembryonic Antigen-Related Cellular Adhesion Molecule 1-Dependent Inhibition of T Cell Responses
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Screening and Identification of Key Biomarkers in Clear Cell Renal Cell Carcinoma Based on Bioinformatics Analysis
bioRxiv preprint doi: https://doi.org/10.1101/2020.12.21.423889; this version posted December 23, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. Screening and identification of key biomarkers in clear cell renal cell carcinoma based on bioinformatics analysis Basavaraj Vastrad1, Chanabasayya Vastrad*2 , Iranna Kotturshetti 1. Department of Biochemistry, Basaveshwar College of Pharmacy, Gadag, Karnataka 582103, India. 2. Biostatistics and Bioinformatics, Chanabasava Nilaya, Bharthinagar, Dharwad 580001, Karanataka, India. 3. Department of Ayurveda, Rajiv Gandhi Education Society`s Ayurvedic Medical College, Ron, Karnataka 562209, India. * Chanabasayya Vastrad [email protected] Ph: +919480073398 Chanabasava Nilaya, Bharthinagar, Dharwad 580001 , Karanataka, India bioRxiv preprint doi: https://doi.org/10.1101/2020.12.21.423889; this version posted December 23, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. Abstract Clear cell renal cell carcinoma (ccRCC) is one of the most common types of malignancy of the urinary system. The pathogenesis and effective diagnosis of ccRCC have become popular topics for research in the previous decade. In the current study, an integrated bioinformatics analysis was performed to identify core genes associated in ccRCC. An expression dataset (GSE105261) was downloaded from the Gene Expression Omnibus database, and included 26 ccRCC and 9 normal kideny samples. Assessment of the microarray dataset led to the recognition of differentially expressed genes (DEGs), which was subsequently used for pathway and gene ontology (GO) enrichment analysis. -

Propranolol-Mediated Attenuation of MMP-9 Excretion in Infants with Hemangiomas
Supplementary Online Content Thaivalappil S, Bauman N, Saieg A, Movius E, Brown KJ, Preciado D. Propranolol-mediated attenuation of MMP-9 excretion in infants with hemangiomas. JAMA Otolaryngol Head Neck Surg. doi:10.1001/jamaoto.2013.4773 eTable. List of All of the Proteins Identified by Proteomics This supplementary material has been provided by the authors to give readers additional information about their work. © 2013 American Medical Association. All rights reserved. Downloaded From: https://jamanetwork.com/ on 10/01/2021 eTable. List of All of the Proteins Identified by Proteomics Protein Name Prop 12 mo/4 Pred 12 mo/4 Δ Prop to Pred mo mo Myeloperoxidase OS=Homo sapiens GN=MPO 26.00 143.00 ‐117.00 Lactotransferrin OS=Homo sapiens GN=LTF 114.00 205.50 ‐91.50 Matrix metalloproteinase‐9 OS=Homo sapiens GN=MMP9 5.00 36.00 ‐31.00 Neutrophil elastase OS=Homo sapiens GN=ELANE 24.00 48.00 ‐24.00 Bleomycin hydrolase OS=Homo sapiens GN=BLMH 3.00 25.00 ‐22.00 CAP7_HUMAN Azurocidin OS=Homo sapiens GN=AZU1 PE=1 SV=3 4.00 26.00 ‐22.00 S10A8_HUMAN Protein S100‐A8 OS=Homo sapiens GN=S100A8 PE=1 14.67 30.50 ‐15.83 SV=1 IL1F9_HUMAN Interleukin‐1 family member 9 OS=Homo sapiens 1.00 15.00 ‐14.00 GN=IL1F9 PE=1 SV=1 MUC5B_HUMAN Mucin‐5B OS=Homo sapiens GN=MUC5B PE=1 SV=3 2.00 14.00 ‐12.00 MUC4_HUMAN Mucin‐4 OS=Homo sapiens GN=MUC4 PE=1 SV=3 1.00 12.00 ‐11.00 HRG_HUMAN Histidine‐rich glycoprotein OS=Homo sapiens GN=HRG 1.00 12.00 ‐11.00 PE=1 SV=1 TKT_HUMAN Transketolase OS=Homo sapiens GN=TKT PE=1 SV=3 17.00 28.00 ‐11.00 CATG_HUMAN Cathepsin G OS=Homo -

Supplementary Table 1: Adhesion Genes Data Set
Supplementary Table 1: Adhesion genes data set PROBE Entrez Gene ID Celera Gene ID Gene_Symbol Gene_Name 160832 1 hCG201364.3 A1BG alpha-1-B glycoprotein 223658 1 hCG201364.3 A1BG alpha-1-B glycoprotein 212988 102 hCG40040.3 ADAM10 ADAM metallopeptidase domain 10 133411 4185 hCG28232.2 ADAM11 ADAM metallopeptidase domain 11 110695 8038 hCG40937.4 ADAM12 ADAM metallopeptidase domain 12 (meltrin alpha) 195222 8038 hCG40937.4 ADAM12 ADAM metallopeptidase domain 12 (meltrin alpha) 165344 8751 hCG20021.3 ADAM15 ADAM metallopeptidase domain 15 (metargidin) 189065 6868 null ADAM17 ADAM metallopeptidase domain 17 (tumor necrosis factor, alpha, converting enzyme) 108119 8728 hCG15398.4 ADAM19 ADAM metallopeptidase domain 19 (meltrin beta) 117763 8748 hCG20675.3 ADAM20 ADAM metallopeptidase domain 20 126448 8747 hCG1785634.2 ADAM21 ADAM metallopeptidase domain 21 208981 8747 hCG1785634.2|hCG2042897 ADAM21 ADAM metallopeptidase domain 21 180903 53616 hCG17212.4 ADAM22 ADAM metallopeptidase domain 22 177272 8745 hCG1811623.1 ADAM23 ADAM metallopeptidase domain 23 102384 10863 hCG1818505.1 ADAM28 ADAM metallopeptidase domain 28 119968 11086 hCG1786734.2 ADAM29 ADAM metallopeptidase domain 29 205542 11085 hCG1997196.1 ADAM30 ADAM metallopeptidase domain 30 148417 80332 hCG39255.4 ADAM33 ADAM metallopeptidase domain 33 140492 8756 hCG1789002.2 ADAM7 ADAM metallopeptidase domain 7 122603 101 hCG1816947.1 ADAM8 ADAM metallopeptidase domain 8 183965 8754 hCG1996391 ADAM9 ADAM metallopeptidase domain 9 (meltrin gamma) 129974 27299 hCG15447.3 ADAMDEC1 ADAM-like, -

The Regulation of Interleukin 7 Receptor Alpha Internalization, Recycling and Degradation by IL-7
Universidade de Lisboa Faculdade de Medicina Unidade de Biologia do Cancro, Instituto de Medicina Molecular The regulation of Interleukin 7 receptor alpha internalization, recycling and degradation by IL-7 - Possible implications in T-cell homeostasis, migration and leukaemogenesis - Catarina Martins de Oliveira Henriques Doutoramento em Ciências Biomédicas For the degree of Doctor of Philosophy 2009 Universidade de Lisboa Faculdade de Medicina Unidade de Biologia do Cancro, Instituto de Medicina Molecular The regulation of Interleukin 7 receptor alpha internalization, recycling and degradation by IL-7 - Possible implications in T-cell homeostasis, migration and leukaemogenesis - Catarina Martins de Oliveira Henriques (Recipient of a scholarship- SFRH7BD/21940/2005 from Fundação para a Ciência e Tecnologia) Tese orientada pelo Doutor João T. Barata, Prof Doutor.Gerard Graham e Prof. Doutora Leonor Parreira Doutoramento em Ciências Biomédicas, especialidade em Ciências Biopatológicas For the degree of Doctor of Philosophy 2009 As opiniões expressas são da exclusiva responsabilidade do seu autor A impressão desta dissertação foi aprovada pela Comissão Coordenadora do Conselho Científico da Faculdade de Medicina de Lisboa em reunião de 13 de Outubro de 2009. Para a Prof. Filomena Mota. Sem o seu apoio e inspiração há muitos anos atrás, eu não seria hoje uma bióloga nem esta tese teria alguma vez existido… Table of Contents Table of contents……………………………………………………….………………….................. i Aknowledgements……………………………………………………………………………………………. -

Combination Immune Therapy
Combination Immune Therapy Translational Medicine Plenary SWOG April 17, 2017 San Francisco T‐cell Activation, Proliferation, and Function is Controlled by Multiple Agonist and Antagonist Signals 1. Co‐stimulation via CD28 ligation 2. CTLA‐4 ligation on activated T 3. T cell function in tissue is subject transduces T cell activating signals cells down‐regulates T cell to feedback inhibition responses T cell Functional Block T cell Activation T cell Activation T cell Proliferative Block T cell T cell APC T cell CTLA‐4 T cell CTLA‐4 TCR TCR PD‐1 PD‐1 PD‐1 IFN‐gamma TCR CD28 Cytokines TCR CD4 MHC MHC PD‐‐L1 MHC B7 MHC CD28 B7 PD‐‐L1 Tumor or Tumor or APC APC Immune cell B7 immune cell APC NK Presence of PD-L1 or TILs1 PPD-L1 /TIL+ PD-L1/TIL + + PD-L1 /TIL+ + PD-L1 /TIL PD-L1 /TIL D-L1 /TIL+ Schalper and Rimm, Yale University NSCLC PD-L1/TIL 45% 17% 26% 12% Type 1 Type 2 Type 3 Type 4 45% 41% 13% 1% Melanoma45% 17% 26% 12% Taube et al Type 1 Type 2 Type 3 Type 4 Tumor‐specific T cells are contained in the PD‐1+ TIL population and are functional after in vitro culture Spectrum of PD‐1/PD‐L1 Antagonist Activity Active • Melanoma Minimal to no activity • Renal cancer (clear cell and non‐clear cell) • Prostate cancer • NSCLC – adenocarcinoma and squamous cell • MMR+ (MSS) colon cancer • Small cell lung cancer • Myeloma • Head and neck cancer • Pancreatic cancer • Gastric and gastroesophageal junction • MMR‐repair deficient tumors (colon, cholangiocarcinoma) • Bladder • Triple negative breast cancer • Ovarian Major PD‐1/PD‐L1 antagonists • Hepatocellular -
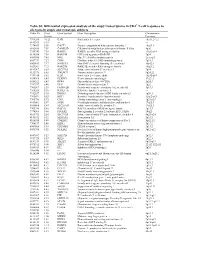
Table S1| Differential Expression Analysis of the Atopy Transcriptome
Table S1| Differential expression analysis of the atopy transcriptome in CD4+ T-cell responses to allergens in atopic and nonatopic subjects Probe ID S.test Gene Symbol Gene Description Chromosome Statistic Location 7994280 10.32 IL4R Interleukin 4 receptor 16p11.2-12.1 8143383 8.95 --- --- --- 7974689 8.50 DACT1 Dapper, antagonist of beta-catenin, homolog 1 14q23.1 8102415 7.59 CAMK2D Calcium/calmodulin-dependent protein kinase II delta 4q26 7950743 7.58 RAB30 RAB30, member RAS oncogene family 11q12-q14 8136580 7.54 RAB19B GTP-binding protein RAB19B 7q34 8043504 7.45 MAL Mal, T-cell differentiation protein 2cen-q13 8087739 7.27 CISH Cytokine inducible SH2-containing protein 3p21.3 8000413 7.17 NSMCE1 Non-SMC element 1 homolog (S. cerevisiae) 16p12.1 8021301 7.15 RAB27B RAB27B, member RAS oncogene family 18q21.2 8143367 6.83 SLC37A3 Solute carrier family 37 member 3 7q34 8152976 6.65 TMEM71 Transmembrane protein 71 8q24.22 7931914 6.56 IL2R Interleukin 2 receptor, alpha 10p15-p14 8014768 6.43 PLXDC1 Plexin domain containing 1 17q21.1 8056222 6.43 DPP4 Dipeptidyl-peptidase 4 (CD26) 2q24.3 7917697 6.40 GFI1 Growth factor independent 1 1p22 7903507 6.39 FAM102B Family with sequence similarity 102, member B 1p13.3 7968236 5.96 RASL11A RAS-like, family 11, member A --- 7912537 5.95 DHRS3 Dehydrogenase/reductase (SDR family) member 3 1p36.1 7963491 5.83 KRT1 Keratin 1 (epidermolytic hyperkeratosis) 12q12-q13 7903786 5.72 CSF1 Colony stimulating factor 1 (macrophage) 1p21-p13 8019061 5.67 SGSH N-sulfoglucosamine sulfohydrolase (sulfamidase) 17q25.3 -

Supplementary Material DNA Methylation in Inflammatory Pathways Modifies the Association Between BMI and Adult-Onset Non- Atopic
Supplementary Material DNA Methylation in Inflammatory Pathways Modifies the Association between BMI and Adult-Onset Non- Atopic Asthma Ayoung Jeong 1,2, Medea Imboden 1,2, Akram Ghantous 3, Alexei Novoloaca 3, Anne-Elie Carsin 4,5,6, Manolis Kogevinas 4,5,6, Christian Schindler 1,2, Gianfranco Lovison 7, Zdenko Herceg 3, Cyrille Cuenin 3, Roel Vermeulen 8, Deborah Jarvis 9, André F. S. Amaral 9, Florian Kronenberg 10, Paolo Vineis 11,12 and Nicole Probst-Hensch 1,2,* 1 Swiss Tropical and Public Health Institute, 4051 Basel, Switzerland; [email protected] (A.J.); [email protected] (M.I.); [email protected] (C.S.) 2 Department of Public Health, University of Basel, 4001 Basel, Switzerland 3 International Agency for Research on Cancer, 69372 Lyon, France; [email protected] (A.G.); [email protected] (A.N.); [email protected] (Z.H.); [email protected] (C.C.) 4 ISGlobal, Barcelona Institute for Global Health, 08003 Barcelona, Spain; [email protected] (A.-E.C.); [email protected] (M.K.) 5 Universitat Pompeu Fabra (UPF), 08002 Barcelona, Spain 6 CIBER Epidemiología y Salud Pública (CIBERESP), 08005 Barcelona, Spain 7 Department of Economics, Business and Statistics, University of Palermo, 90128 Palermo, Italy; [email protected] 8 Environmental Epidemiology Division, Utrecht University, Institute for Risk Assessment Sciences, 3584CM Utrecht, Netherlands; [email protected] 9 Population Health and Occupational Disease, National Heart and Lung Institute, Imperial College, SW3 6LR London, UK; [email protected] (D.J.); [email protected] (A.F.S.A.) 10 Division of Genetic Epidemiology, Medical University of Innsbruck, 6020 Innsbruck, Austria; [email protected] 11 MRC-PHE Centre for Environment and Health, School of Public Health, Imperial College London, W2 1PG London, UK; [email protected] 12 Italian Institute for Genomic Medicine (IIGM), 10126 Turin, Italy * Correspondence: [email protected]; Tel.: +41-61-284-8378 Int. -

Bioinformatics Method Identifies Potential Biomarkers of Dilated Cardiomyopathy in a Human Induced Pluripotent Stem Cell‑Derived Cardiomyocyte Model
EXPERIMENTAL AND THERAPEUTIC MEDICINE 14: 2771-2778, 2017 Bioinformatics method identifies potential biomarkers of dilated cardiomyopathy in a human induced pluripotent stem cell‑derived cardiomyocyte model YU ZHUANG1, YU-JIA GONG2, BEI-FEN ZHONG3, YI ZHOU1 and LI GONG4 1Department of Cardiovascular Surgery, Shanghai First People's Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai 200080; 2Stomatology Faculty, School of Medicine, Nantong University, Nantong, Jiangsu 226000; 3Department of Urology, Shanghai First People's Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai 200080; 4Department of Cardiothoracic Surgery, The Affiliated Huai'an Hospital of Xuzhou Medical University, Huai'an, Jiangsu 223002, P.R. China Received February 19, 2016; Accepted February 10, 2017 DOI: 10.3892/etm.2017.4850 Abstract. Dilated cardiomyopathy (DCM) is the most common crucial nodes in module 2, which were linked to each other. type of cardiomyopathy that account for the majority of heart In conclusion, several potential biomarkers for DCM were failure cases. The present study aimed to reveal the under- identified, such as MMP2, FLT1, CDH1, ITGB6, COL6A3, lying molecular mechanisms of DCM and provide potential COL6A1, LAMC2, PENK and APLNR. These genes may serve biomarkers for detection of this condition. The public dataset significant roles in DCM via involvement of various BPs, such of GSE35108 was downloaded, and 4 normal induced pluripo- as blood vessel and vasculature development and cell adhe- tent stem cell (iPSC)-derived cardiomyocytes (N samples) and sion, and the ECM-receptor interaction pathway. 4 DCM iPSC-derived cardiomyocytes (DCM samples) were utilized. Raw data were preprocessed, followed by identifica- Introduction tion of differentially expressed genes (DEGs) between N and DCM samples. -

The Immunomodulatory CEA Cell Adhesion Molecule 6 (CEACAM6/Cd66c) Is a Candidate Receptor for the Influenza a Virus
bioRxiv preprint doi: https://doi.org/10.1101/104026; this version posted January 30, 2017. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. 1 The immunomodulatory CEA cell adhesion molecule 6 (CEACAM6/CD66c) is a 2 candidate receptor for the influenza A virus 3 Shah Kamranur Rahmana *, Mairaj Ahmed Ansarib, Pratibha Gaurc, Imtiyaz Ahmada, 4 Chandrani Chakravartya,d, Dileep Kumar Vermaa, Sanjay Chhibbere, Naila Nehalf, 5 Shanmugaapriya Sellathanbyd, Dagmar Wirthc, Gulam Warisb and Sunil K. Lala,g # 6 7 Virology Group, International Centre for Genetic Engineering & Biotechnology, New Delhi, 8 Indiaa. 9 Department of Microbiology and Immunology, H. M. Bligh Cancer Research Laboratories, 10 Rosalind Franklin University of Medicine and Science, Chicago Medical School, North 11 Chicago, Illinois, USAb. 12 Helmholtz Centre for Infection Research, Braunschweig, Germanyc. 13 Department of Biomedical Science, Bharathidasan University, Trichy, Indiad. 14 Microbiology Department, Panjab University, Chandigarh, Indiae. 15 Career Institute of Medical & Dental Sciences and Hospital, Lucknow, Indiaf. 16 School of Science, Monash University, Selangor DE, Malaysiag. 17 18 Running Head: Protein receptor for Influenza A Virus 19 20 # Corresponding author: Professor of Microbiology, School of Science, Monash University, 21 47500 Bandar Sunway, Selangor DE, Malaysia. 22 Email: [email protected]; Telephone: (+603) 551 59606 23 24 * Current address: Department of Pathogen Molecular Biology, London School of Hygiene & 25 Tropical Medicine, Keppel Street, London WC1E 7HT, United Kingdom. 26 1 bioRxiv preprint doi: https://doi.org/10.1101/104026; this version posted January 30, 2017. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. -

(Lilrs) on Human Neutrophils: Modulators of Infection and Immunity
MINI REVIEW published: 13 May 2020 doi: 10.3389/fimmu.2020.00857 Leukocyte Immunoglobulin-Like Receptors (LILRs) on Human Neutrophils: Modulators of Infection and Immunity Alexander L. Lewis Marffy and Alex J. McCarthy* MRC Centre for Molecular Bacteriology and Infection, Imperial College London, London, United Kingdom Neutrophils have a crucial role in defense against microbes. Immune receptors allow neutrophils to sense their environment, with many receptors functioning to recognize signs of infection and to promote antimicrobial effector functions. However, the neutrophil Edited by: Nicole Thielens, response must be tightly regulated to prevent excessive inflammation and tissue damage, UMR5075 Institut de Biologie and regulation is achieved by expression of inhibitory receptors that can raise activation Structurale (IBS), France thresholds. The leukocyte immunoglobulin-like receptor (LILR) family contain activating Reviewed by: and inhibitory members that can up- or down-regulate immune cell activity. New ligands Debby Burshtyn, University of Alberta, Canada and functions for LILR continue to emerge. Understanding the role of LILR in neutrophil Tamás Laskay, biology is of general interest as they can activate and suppress antimicrobial responses University of Lübeck, Germany of neutrophils and because several human pathogens exploit these receptors for immune *Correspondence: Alex J. McCarthy evasion. This review focuses on the role of LILR in neutrophil biology. We focus on the [email protected] current knowledge of LILR expression -

Systemic Surfaceome Profiling Identifies Target Antigens for Immune-Based Therapy in Subtypes of Advanced Prostate Cancer
Systemic surfaceome profiling identifies target PNAS PLUS antigens for immune-based therapy in subtypes of advanced prostate cancer John K. Leea,b,c, Nathanael J. Bangayand, Timothy Chaie, Bryan A. Smithf, Tiffany E. Parivaf, Sangwon Yung, Ajay Vashishth, Qingfu Zhangi,j, Jung Wook Parkf, Eva Coreyk, Jiaoti Huangi, Thomas G. Graeberc,d,l,m, James Wohlschlegelh, and Owen N. Wittec,d,f,n,o,1 aDivision of Hematology and Oncology, Department of Medicine, University of California, Los Angeles, CA 90095; bInstitute of Urologic Oncology, Department of Urology, University of California, Los Angeles, CA 90095; cJonsson Comprehensive Cancer Center, University of California, Los Angeles, CA 90095; dDepartment of Molecular and Medical Pharmacology, University of California, Los Angeles, CA 90095; eStanford University School of Medicine, Palo Alto, CA 94305; fDepartment of Microbiology, Immunology, and Medical Genetics, University of California, Los Angeles, CA 90095; gYale School of Medicine, New Haven, CT 06510; hDepartment of Biological Chemistry, University of California, Los Angeles, CA 90095; iDepartment of Pathology, Duke University School of Medicine, Durham, NC 27708; jDepartment of Pathology, China Medical University, 110001 Shenyang, People’s Republic of China; kDepartment of Urology, University of Washington School of Medicine, Seattle, WA 98195; lCrump Institute for Molecular Imaging, University of California, Los Angeles, CA 90095; mUCLA Metabolomics Center, University of California, Los Angeles, CA 900095; nParker Institute for Cancer -

Supplemental Text and Figures
Supplemental Materials and Methods Experimental bone metastasis assay Primary PCa cells were sorted by GFP marker from mTmG+ tumors, and 105 cells in 20μL PBS were injected using Hamilton syringe into the tibia of 6-week old NSG mice. Mice were monitored biweekly for moribund signs for euthanasia and organ harvest. Noninvasive mouse and ex vivo imaging MRI imaging with Bruker ICON and fluorescence imaging of fresh organs with metastasis enumeration were recently described (Lu et al. 2017). Primary prostate cell sphere formation assay Isolate of primary cells from prostate, culture and counting of prostatospheres on Matrigel were performed as described (Lukacs et al. 2010). For organoid culture assay, we followed a published matrigel embedding method (Chua et al. 2014). RNA-Seq and differential gene expression Total RNA was isolated from prostate tumors using Direct-zol RNA MiniPrep Kit (Zymo Research) and processed for stranded total RNA-Seq using Illumina HiSeq 4000 at Sequencing and Microarray Facility at MD Anderson Cancer Center. The differential expression analysis was performed using the DESeq2 package of R. P-values obtained after multiple binomial tests were adjusted using BH method. Significant genes are defined by using a cut-off of 0.05 on the BH corrected p-value and an absolute log2 fold change value of at least 1.5. Histology and western blot H&E stain, immunohistochemical (IHC) and western blot were performed as previously described (Ding et al. 2011; Wang et al. 2016). Primary antibodies for IHC include Ki67 (Fisher, RM-9106-S1), cleaved caspase 3 (Cell Signaling Technology aka CST, 9661), cyclin D1 (Fisher, clone SP4), TGFBR2 (Abcam, ab61213), BMPR2 (Abcam, ab130206), AR (EMD Millipore, 06-680), phospho- Akt (CST, 4060), GFP (CST, 2956), E-Cadherin (CST, 14472).