CLINICAL GUIDELINES Spine Surgery
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Modified Plate-Only Open-Door Laminoplasty Versus Laminectomy and Fusion for the Treatment of Cervical Stenotic Myelopathy
n Feature Article Modified Plate-only Open-door Laminoplasty Versus Laminectomy and Fusion for the Treatment of Cervical Stenotic Myelopathy LILI YANG, MD; YIFEI GU, MD; JUEQIAN SHI, MD; RUI GAO, MD; YANG LIU, MD; JUN LI, MD; WEN YUAN, MD, PHD abstract Full article available online at Healio.com/Orthopedics. Search: 20121217-23 The purpose of this study was to compare modified plate-only laminoplasty and lami- nectomy and fusion to confirm which of the 2 surgical modalities could achieve a better decompression outcome and whether a significant difference was found in postopera- tive complications. Clinical data were retrospectively reviewed for 141 patients with cervical stenotic myelopathy who underwent plate-only laminoplasty and laminectomy and fusion between November 2007 and June 2010. The extent of decompression was assessed by measuring the cross-sectional area of the dural sac and the distance of spinal cord drift at the 3 most narrowed levels on T2-weighted magnetic resonance imaging. Clinical outcomes and complications were also recorded and compared. Significant en- largement of the dural sac area and spinal cord drift was achieved and well maintained in both groups, but the extent of decompression was greater in patients who underwent Figure: T2-weighted magnetic resonance image laminectomy and fusion; however, a greater decompression did not seem to produce a showing the extent of decompression assessed by better clinical outcome. No significant difference was observed in Japanese Orthopaedic measuring the cross-sectional area of the dural sac Association and Nurick scores between the 2 groups. Patients who underwent plate-only (arrow). laminoplasty showed a better improvement in Neck Dysfunction Index and visual ana- log scale scores. -

Anterior Reconstruction Techniques for Cervical Spine Deformity
Neurospine 2020;17(3):534-542. Neurospine https://doi.org/10.14245/ns.2040380.190 pISSN 2586-6583 eISSN 2586-6591 Review Article Anterior Reconstruction Techniques Corresponding Author for Cervical Spine Deformity Samuel K. Cho 1,2 1 1 1 https://orcid.org/0000-0001-7511-2486 Murray Echt , Christopher Mikhail , Steven J. Girdler , Samuel K. Cho 1Department of Orthopedics, Icahn School of Medicine at Mount Sinai, New York, NY, USA Department of Orthopaedics, Icahn 2 Department of Neurological Surgery, Montefiore Medical Center/Albert Einstein College of Medicine, Bronx, School of Medicine at Mount Sinai, 425 NY, USA West 59th Street, 5th Floor, New York, NY, USA E-mail: [email protected] Cervical spine deformity is an uncommon yet severely debilitating condition marked by its heterogeneity. Anterior reconstruction techniques represent a familiar approach with a range Received: June 24, 2020 of invasiveness and correction potential—including global or focal realignment in the sagit- Revised: August 5, 2020 tal and coronal planes. Meticulous preoperative planning is required to improve or prevent Accepted: August 17, 2020 neurologic deterioration and obtain satisfactory global spinal harmony. The ability to per- form anterior only reconstruction requires mobility of the opposite column to achieve cor- rection, unless a combined approach is planned. Anterior cervical discectomy and fusion has limited focal correction, but when applied over multiple levels there is a cumulative ef- fect with a correction of approximately 6° per level. Partial or complete corpectomy has the ability to correct sagittal deformity as well as decompress the spinal canal when there is an- terior compression behind the vertebral body. -

2019 Spine Coding Basics
2019 Spine Coding Basics Presenter: Kerri Larson, CPC Directory of Coding and Audit Services 2019 Spine Surgery 01 Spine Surgery Terminology & Anatomy 02 Spine Procedures 03 Case Study 04 Diagnosis 05 Q & A Spine Surgery Terminology & Anatomy Spine Surgery Terminology & Anatomy Term Definition Arthrodesis Fusion, or permanent joining, of a joint, or point of union of two musculoskeletal structures, such as two bones Surgical procedure that replaces missing bone with material from the patient's own body, or from an artificial, synthetic, or Bone grafting natural substitute Corpectomy Surgical excision of the main body of a vertebra, one of the interlocking bones of the back. Cerebrospinal The protective body fluid present in the dura, the membrane covering the brain and spinal cord fluid or CSF Decompression A procedure to remove pressure on a structure. Diskectomy, Surgical removal of all or a part of an intervertebral disc. discectomy Dura Outermost of the three layers that surround the brain and spinal cord. Electrode array Device that contains multiple plates or electrodes. Electronic pulse A device that produces low voltage electrical pulses, with a regular or intermittent waveform, that creates a mild tingling or generator or massaging sensation that stimulates the nerve pathways neurostimulator Spine Surgery Terminology & Anatomy Term Definition The space that surrounds the dura, which is the outermost layer of membrane that surrounds the spinal canal. The epidural space houses the Epidural space spinal nerve roots, blood and lymphatic vessels, and fatty tissues . Present inside the skull but outside the dura mater, which is the thick, outermost membrane covering the brain or within the spine but outside Extradural the dural sac enclosing the spinal cord, nerve roots and spinal fluid. -

Analysis of the Cervical Spine Alignment Following Laminoplasty and Laminectomy
Spinal Cord (1999) 37, 20± 24 ã 1999 International Medical Society of Paraplegia All rights reserved 1362 ± 4393/99 $12.00 http://www.stockton-press.co.uk/sc Analysis of the cervical spine alignment following laminoplasty and laminectomy Shunji Matsunaga1, Takashi Sakou1 and Kenji Nakanisi2 1Department of Orthopaedic Surgery, Faculty of Medicine, Kagoshima University; 2Department of Mechanical Engineering, Faculty of Engineering, Kagoshima University, Sakuragaoka, Kagoshima, Japan Very little detailed biomechanical examination of the alignment of the cervical spine following laminoplasty has been reported. We performed a comparative study regarding the buckling- type alignment that follows laminoplasty and laminectomy to know the mechanical changes in the alignment of the cervical spine. Lateral images of plain roentgenograms of the cervical spine were put into a computer and examined using a program we developed for analysis of the buckling-type alignment. Sixty-four patients who underwent laminoplasty and 37 patients who underwent laminectomy were reviewed retrospectively. The subjects comprised patients with cervical spondylotic myelopathy (CSM) and those with ossi®cation of the posterior longitudinal ligament (OPLL). The postoperative observation period was 6 years and 7 months on average after laminectomy, and 5 years and 6 months on average following laminoplasty. Development of the buckling-type alignment was found in 33% of patients following laminectomy and only 6% after laminoplasty. Development of buckling-type alignment following laminoplasty appeared markedly less than following laminectomy in both CSM and OPLL patients. These results favor laminoplasty over laminectomy from the aspect of mechanics. Keywords: laminoplasty; laminectomy; buckling; kyphosis; swan-neck deformity Introduction In 1930, Eiselberg1 reported a case of postoperative of postoperative abnormal alignment from the aspect kyphosis of the spine following laminectomy from the of the presence or absence of buckling-type alignment. -

Spinal Interventional Pain Management and Lumbar Spine Surgery
Spinal Interventional Pain Management and Lumbar Spine Surgery Policy Number: Original Effective Date: MM.06.024 01/01/2014 Line(s) of Business: Current Effective Date: HMO; PPO; QUEST Integration 12/15/2017 Section: Surgery; Medicine Place(s) of Service: Office; Outpatient; Inpatient I. Description The following spinal interventional pain management and lumbar spine surgery procedures require precertification through Magellan Hawaii, formally known as National Imaging Associates, Inc. (NIA): A. Spinal Epidural Injections B. Paravertebral Facet Joint Denervation (radiofrequency neurolysis) C. Paravertebral Facet Joint Injections or Blocks D. Sacroiliac joint injections E. Lumbar Spinal Fusion Surgery II. Administrative Guidelines A. The ordering physician can obtain precertification or consult with Magellan Hawaii by accessing their website at http://www.radmd.com/ or by calling 1 (866) 306-9729, from 6 a.m. to 6 p.m., weekdays, Hawaii Time. Refer to the e-library for instructions on navigating the radmd.com website (RadMD Get Started) and requesting precertification/checking the status of a request (RadMD QuickStart). B. For access to the latest clinical guidelines used for precertification, go to www.radmd.com and click on the link entitled View Clinical Guidelines. C. For interventional pain management procedures (epidural injections, facet joint denervation neurolysis, facet joint injections and sacroiliac joint injections), if more than one procedure is planned, a separate precertification number must be obtained for each procedure planned. D. For spinal surgeries (lumbar fusions, lumbar decompressions, and lumbar microdiscectomy), one precertification number should be obtained for the most invasive surgery to be performed. E. Precertification requirements for injection procedures apply only to office and outpatient services (POS 11, 22, or 24). -

Lumbar Laminectomy
Patient Education Lumbar Laminectomy Description The spine consists of five separate divisions: cervical (seven vertebrae), thoracic (12 vertebrae), lumbar (five vertebrae), the sacrum, and the coccyx. Each vertebra, interlocks with the segment above and below it through the superior and inferior articular processes. Between each vertebra is an intervertebral disc that provides cushioning for the spine. The lamina and pedicle, along with the vertebral body, provide the borders that create the spinal canal, which the spinal cord runs through to transmit nerve signals. There are several different scenarios or conditions that may produce symptoms that would lead your physician to further Medical Illustration © 2016 Nucleus Medical Art, Inc. investigate, and possibly recommend this surgery. Stenosis causing Radicular Pain Spinal stenosis is the narrowing of the articular spaces within the spine; this may impinge on the nerves or the spinal cord. This is a degenerative process and may eventually lead to further changes on the spine over time. Radicular symptoms are pain, numbness, weakness, tingling, etc., that radiate along a specific nerve root (or dermatome) to other parts of the body outside of the spine. Surgical correction of this problem may include a minimally invasive decompression (shaving bone away to create more space around the nerve), often referred to as laminectomy (removing part or all of the lamina in order to provide more space and relieve impingement). In some cases, movement of one vertebrae slipping against another (spondylolisthesis), may require a vertebral fusion. This may be performed open vs. minimally invasive. Disc Herniation Herniation of the intervertebral disc may be due to an acute traumatic incident. -

Modern Advances in Joint Replacement and Rapid Recovery
Modern Advances in Joint Replacement and Rapid Recovery UCSF Osher Mini-Med School Lecture Series Jeffrey Barry, M.D. Assistant Professor of Orthopaedic Surgery Division of Adult Reconstruction University of California, San Francisco Disclosures . No relevant disclosures to this talk About Me . Bay Area Native . UCSF - U Can Stay Forever Outline . Burden of Disease and Epidemiology . The Basics of Hip and Knee Replacement . What’s improving over the last decade - Longevity - Pain Management - Hospital Stay - Thromboembolism prophylaxis - Risk Reduction Question What is the most common inpatient surgery performed in the US? 1. Percutaneous coronary angioplasty 2. Total hip replacement 3. Lumbar Laminectomy 4. Appendectomy 5. Total knee replacement Burden of Disease . Arthritis = most common cause of disability in the US . 22.7% of adults have doctor-diagnosed arthritis - 43.2% of patients with arthritis report activity limitations due to disease . By 2030: - 3.5 million TKA (673%) - 570,000 THA (174%) . Curve updated 2014 – just as predicted! Causes of Increased Utilization . Aging Population . Patients receiving arthroplasty at a younger age - Improvements in technology - Obesity Why Replace a Joint? Arthritis arthro – joint itis – inflammation What is Arthritis – Disease of Cartilage . Cartilage Degeneration - Pain - Limp - Swelling - Loss of range of motion - Eventual deformity Arthritis Affects on Your Life • Quality of Life • Independence • Movement, Walking, Exercise • Self-image • Self-esteem • Family Life • Sleep • Everything and Everybody Causes of Arthritis . Osteoarthritis - “wear and tear” . Inflammatory arthritis . Trauma, old fractures . Infection . Osteonecrosis - “lack of oxygen to the bone” . Childhood/ developmental disease Diagnosis . Clinical Symptoms + Radiographic . Radiographs – Standing or Weight bearing! . MRI is RARELY needed!!! - Expensive - Brings in other issues - Unnecessary treatment - Unnecessary explanations Knee Arthritis . -

Automated Percutaneous and Endoscopic Discectomy
Corporate Medical Policy Automated Percutaneous and Endoscopic Discectomy File Name: percutaneous_discectomy Origination: 9/1991 Last CAP Review: 5/2021 Next CAP Review: 5/2022 Last Review: 5/2021 Description of Procedure or Service Surgical management of herniated intervertebral discs most commonly involves discectomy or microdiscectomy, performed manually through an open incision. Automated percutaneous discectomy involves placement of a probe within the intervertebral disc under image guidance with aspiration of disc material using a suction cutting device. Removal of disc herniations under endoscopic visualization is also being investigated. Endoscopic discectomy involves the percutaneous placement of a working channel under image guidance, followed by visualization of the working space and instruments through an endoscope, and aspiration of disc material. Back pain or radiculopathy related to herniated discs is an extremely common condition and a frequent cause of chronic disability. Although many cases of acute low back pain and radiculopathy will resolve with conservative care, a surgical decompression is often considered when the pain is unimproved after several months and is clearly neuropathic in origin, resulting from irritation of the nerve roots. Open surgical treatment typically consists of discectomy, in which the extruding disc material is excised. When performed with an operating microscope the procedure is known as microdiscectomy. Minimally invasive options have also been researched, in which some portion of the disc is removed or ablated, although these techniques are not precisely targeted at the offending extruding disc material. Ablative techniques include laser discectomy and radiofrequency (RF) decompression. In addition, intradiscal electrothermal annuloplasty is another minimally invasive approach to low back pain. -
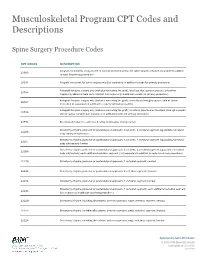
Musculoskeletal Program CPT Codes and Descriptions
Musculoskeletal Program CPT Codes and Descriptions Spine Surgery Procedure Codes CPT CODES DESCRIPTION Allograft, morselized, or placement of osteopromotive material, for spine surgery only (List separately in addition 20930 to code for primary procedure) 20931 Allograft, structural, for spine surgery only (List separately in addition to code for primary procedure) Autograft for spine surgery only (includes harvesting the graft); local (eg, ribs, spinous process, or laminar 20936 fragments) obtained from same incision (List separately in addition to code for primary procedure) Autograft for spine surgery only (includes harvesting the graft); morselized (through separate skin or fascial 20937 incision) (List separately in addition to code for primary procedure) Autograft for spine surgery only (includes harvesting the graft); structural, bicortical or tricortical (through separate 20938 skin or fascial incision) (List separately in addition to code for primary procedure) 20974 Electrical stimulation to aid bone healing; noninvasive (nonoperative) Osteotomy of spine, posterior or posterolateral approach, 3 columns, 1 vertebral segment (eg, pedicle/vertebral 22206 body subtraction); thoracic Osteotomy of spine, posterior or posterolateral approach, 3 columns, 1 vertebral segment (eg, pedicle/vertebral 22207 body subtraction); lumbar Osteotomy of spine, posterior or posterolateral approach, 3 columns, 1 vertebral segment (eg, pedicle/vertebral 22208 body subtraction); each additional vertebral segment (List separately in addition to code for -
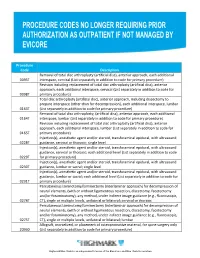
Procedure Codes No Longer Requiring Prior
Procedure Code Description Removal of total disc arthroplasty (artificial disc), anterior approach, each additional 0095T interspace, cervical (List separately in addition to code for primary procedure) Revision including replacement of total disc arthroplasty (artificial disc), anterior approach, each additional interspace, cervical (List separately in addition to code for 0098T primary procedure) Total disc arthroplasty (artificial disc), anterior approach, including discectomy to prepare interspace (other than for decompression), each additional interspace, lumbar 0163T (List separately in addition to code for primary procedure) Removal of total disc arthroplasty, (artificial disc), anterior approach, each additional 0164T interspace, lumbar (List separately in addition to code for primary procedure) Revision including replacement of total disc arthroplasty (artificial disc), anterior approach, each additional interspace, lumbar (List separately in addition to code for 0165T primary procedure) Injection(s), anesthetic agent and/or steroid, transforaminal epidural, with ultrasound 0228T guidance, cervical or thoracic; single level Injection(s), anesthetic agent and/or steroid, transforaminal epidural, with ultrasound guidance, cervical or thoracic; each additional level (List separately in addition to code 0229T for primary procedure) Injection(s), anesthetic agent and/or steroid, transforaminal epidural, with ultrasound 0230T guidance, lumbar or sacral; single level Injection(s), anesthetic agent and/or steroid, transforaminal epidural, -

Biomechanical Evaluation of Relationship of Screw Pullout
ORIGINAL ARTICLE Biomechanical Evaluation of Relationship of Screw Pullout Strength, Insertional Torque, and Bone Mineral Density in the Cervical Spine Charles Alan Reitman, MD, Lyndon Nguyen, and Guy R. Fogel help prevent implant loosening and add rigidity to the plate- Background: Understanding of implant failure mechanisms is im- screw construct. Some screws actually lock to the plate, while portant in the successful utilization of anterior cervical plates. Many in most cases, there is some kind of blocking plate or screw variables influence screw purchase, including the quality of the bone. head expansion to secure the screw to the plate. In these cases, The purpose of this study was to assess the relationship of screw pull- out and screw insertional torque across a wide range of bone mineral the screws are initially placed securely per the surgeon’s own densities (BMDs). perception, in most instances without specific torque control. Screw pullout and stripping (exceeding maximal inser- Methods: A total of 54 cervical vertebrae in 12 cervical spines were tional torque) are possible modes of failure. Some factors af- evaluated for BMD using dual-energy x-ray absorptiometry scan- fecting the pullout strength of a cancellous bone screw are spe- ning. Actual and perceived peak torques of 3.5-mm anterior cervical cific to the screw design and include the major diameter of the screws were determined at each level followed by screw pullout screw, the length of engagement of the thread, and screw strength testing. thread depth and pitch.4 Furthermore, tapping was found to Results: A high correlation was observed between screw pullout reduce pullout force by an average of 8% compared with non- strength and BMD. -

Posterior Foraminotomy Versus Anterior Decompression and Fusion
CLINICAL ARTICLE J Neurosurg Spine 32:344–352, 2020 Posterior foraminotomy versus anterior decompression and fusion in patients with cervical degenerative disc disease with radiculopathy: up to 5 years of outcome from the national Swedish Spine Register Anna MacDowall, MD, PhD,1 Robert F. Heary, MD,2 Marek Holy, MD,3 Lars Lindhagen, PhD,4 and Claes Olerud, MD, PhD1 1Department of Surgical Sciences, Uppsala University Hospital, Uppsala, Sweden; 2Department of Neurological Surgery, Rutgers New Jersey Medical School, Newark, New Jersey; 3Department of Orthopedics, Örebro University Hospital, Örebro; and 4Uppsala Clinical Research Center, Uppsala University, Uppsala, Sweden OBJECTIVE The long-term efficacy of posterior foraminotomy compared with anterior cervical decompression and fusion (ACDF) for the treatment of degenerative disc disease with radiculopathy has not been previously investigated in a population-based cohort. METHODS All patients in the national Swedish Spine Register (Swespine) from January 1, 2006, until November 15, 2017, with cervical degenerative disc disease and radiculopathy were assessed. Using propensity score matching, pa- tients treated with posterior foraminotomy were compared with those undergoing ACDF. The primary outcome measure was the Neck Disability Index (NDI), a patient-reported outcome score ranging from 0% to 100%, with higher scores indicating greater disability. A minimal clinically important difference was defined as > 15%. Secondary outcomes were assessed with additional patient-reported outcome measures (PROMs). RESULTS A total of 4368 patients (2136/2232 women/men) met the inclusion criteria. Posterior foraminotomy was performed in 647 patients, and 3721 patients underwent ACDF. After meticulous propensity score matching, 570 patients with a mean age of 54 years remained in each group.