Gestational Ovarian Choriocarcinoma: a Review of the Literature and Presentation of a Perplexing Case
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
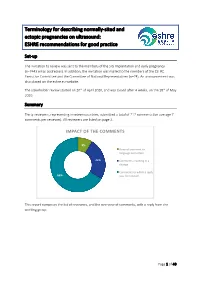
Terminology for Describing Normally-Sited and Ectopic Pregnancies on Ultrasound: ESHRE Recommendations for Good Practice
Terminology for describing normally-sited and ectopic pregnancies on ultrasound: ESHRE recommendations for good practice Set-up The invitation to review was sent to the members of the SIG Implantation and early pregnancy (n=7443 email addresses). In addition, the invitation was mailed to the members of the ESHRE Executive Committee and the Committee of National Representatives (n=74). An announcement was also placed on the eshre.eu website. The stakeholder review started on 20th of April 2020, and was closed after 4 weeks, on the 18th of May 2020. Summary Thirty reviewers, representing nineteen countries, submitted a total of 212 comments (on average 7 comments per reviewer). All reviewers are listed on page 2. IMPACT OF THE COMMENTS 9% General comment or language correction 25% Comments resulting in a change Comments to which a reply 66% was formulated This report comprises the list of reviewers, and the overview of comments, with a reply from the working group. Page 1 of 40 List of reviewers Name Country Organization Masoud Kamrava Iran Steven Goldstein USA Ulrike Metzger France Grigorios Derdelis Greece Luca Savelli Italy Gangaraju Buvaneswari India Mridu Sinha India Onur Erol Turkey Aboubakr Mohamed Elnashar Egypt Jiuzhi Zeng China Carlos Calhaz-Jorge Portugal Dr. Nidaa Qatar Attilio Di Spiezio Sardo Italy Melinda Mitranovici Romania Ali Sami Gurbuz Turkey Philippe Merviel France Roy Farquharson UK Ilan Timor USA Alessandra Pipan United Arab Emirates Company, Taiwan IVF Wen Jui Yang Taiwan Group Center Lorenzo Abad de Velasco Spain Monica Varma India Kumaran Aswathy India Annick Geril Belgium Snezana Vidakovic Serbia Lukasz Polanski, Miriam Baumgarten, Rebecca McKay, Laura Rutherford, Ouma Pillay, Anita Jeyaraj, Kanna Jayaprakasan and Kamal Ojha UK organization Mohamed Shahin UK Mohsen M El-Sayed UK International Society of Ultrasound in Obstetrics and Gynecology (ISUOG) ISUOG European Niche taskforce group of the ESGE and international Prof. -

Sonography in First Trimester Bleeding
Review Sonography in First Trimester Bleeding Manjiri Dighe, MD,1 Carlos Cuevas, MD,1 Mariam Moshiri, MD,1 Theodore Dubinsky, MD,1 Vikram S. Dogra, MD2 1 Department of Radiology, University of Washington Medical Center, Seattle, WA 98195 2 Department of Imaging Sciences, University of Rochester School of Medicine, 601 Elmwood Avenue, Box 648, Rochester, NY 14642 Received 19 April 2007; accepted 1 October 2007 ABSTRACT: Vaginal bleeding is the most common however, the most common cause of bleeding is cause of presentation to the emergency department spotting caused by implantation of the conceptus in the first trimester. Approximately half of patients into the endometrium. A complete assessment of with first trimester vaginal bleeding will lose the preg- the first trimester pregnancy requires correlation nancy. Clinical assessment is difficult, and sonogra- of serum b human chorionic gonadotropin (b- phy is necessary to determine if a normal fetus is hCG) levels with the appearance of the gesta- present and alive and to exclude other causes of bleeding (eg, ectopic or molar pregnancy). Diagnosis tional sac (GS) using sonography. of a normal intrauterine pregnancy not only helps the physician in terms of management but also gives psy- SPONTANEOUS ABORTION chologic relief to the patient. Improved ultrasound technology and high-frequency endovaginal trans- Spontaneous abortion is defined as the termina- ducers have enabled early diagnosis of abnormal and tion of a pregnancy before the twentieth completed ectopic pregnancies, decreasing maternal morbidity week of gestation. Sixty-five percent of spontane- and mortality. The main differential considerations of ous abortions occur in the first 16 weeks of preg- first trimester bleeding are spontaneous abortion, ec- nancy. -

Board-Review-Series-Obstetrics-Gynecology-Pearls.Pdf
ObstetricsandGynecology BOARDREVIEW Third Edition Stephen G. Somkuti, MD, PhD Associate Professor Department of Obstetrics and Gynecology and Reproductive Sciences Temple University School of Medicine School Philadelphia, Pennsylvania Director, The Toll Center for Reproductive Sciences Division of Reproductive Endocrinology Department of Obstetrics and Gynecology Abington Memorial Hospital Abington Reproductive Medicine Abington, Pennsylvania New York Chicago San Francisco Lisbon London Madrid Mexico City Milan New Delhi San Juan Seoul Singapore Sydney Toronto Copyright © 2008 by the McGraw-Hill Companies, Inc. All rights reserved. Manufactured in the United States of America. Except as permitted under the United States Copyright Act of 1976, no part of this publication may be reproduced or distributed in any form or by any means, or stored in a database or retrieval system, without the prior written permission of the publisher. 0-07-164298-6 The material in this eBook also appears in the print version of this title: 0-07-149703-X. All trademarks are trademarks of their respective owners. Rather than put a trademark symbol after every occurrence of a trademarked name, we use names in an editorial fashion only, and to the benefit of the trademark owner, with no intention of infringement of the trademark. Where such designations appear in this book, they have been printed with initial caps. McGraw-Hill eBooks are available at special quantity discounts to use as premiums and sales promotions, or for use in corporate training programs. For more information, please contact George Hoare, Special Sales, at [email protected] or (212) 904-4069. TERMS OF USE This is a copyrighted work and The McGraw-Hill Companies, Inc. -

Original Article Advanced Abdominal Pregnancy: an Increasingly Challenging Clinical Concern for Obstetricians
Int J Clin Exp Pathol 2014;7(9):5461-5472 www.ijcep.com /ISSN:1936-2625/IJCEP0001373 Original Article Advanced abdominal pregnancy: an increasingly challenging clinical concern for obstetricians Ke Huang1, Lei Song1, Longxia Wang2, Zhiying Gao1, Yuanguang Meng1, Yanping Lu1 Departments of 1Gynecology and Obstetrics, 2Ultrasonography, The PLA General Hospital, Beijing City, China Received July 9, 2014; Accepted August 21, 2014; Epub August 15, 2014; Published September 1, 2014 Abstract: Advanced abdominal pregnancy is rare. The low incidence, high misdiagnosis rate, and lack of specific clinical signs and symptoms explain the fact that there are no standard diagnostic and treatment options available for advanced abdominal pregnancy. We managed a case of abdominal pregnancy in a woman who was pregnant for the first time. This case was further complicated by a concurrent singleton intrauterine pregnancy; the twin preg- nancy was not detected until 20 weeks of pregnancy. The case was confirmed at 26 weeks gestational age using MRI to be an abdominal combined with intrauterine pregnancy. The pregnancy was terminated by cesarean section at 33 + 5 weeks gestation. We collected the relevant data of the case while reviewing the advanced abdominal pregnancy-related English literature in the Pubmed, Proquest, and OVID databases. We compared and analyzed the pregnancy history, gestational age when the diagnosis was confirmed, the placental colonization position, the course of treatment and surgical processes, related concurrency rate, post-operative drug treatment programs, and follow-up results with the expectation to provide guidance for other physicians who might encounter similar cases. Keywords: Advanced abdominal pregnancy, obstetricians, clinical Introduction abdominal pregnancies [8-10]. -

Ovarian Ectopic Pregnancy with Newborn at Term: a Case Report
Case Report Glob J Reprod Med Volume 5 Issue 2 - July 2018 Copyright © All rights are reserved by Quirino I DOI: 10.19080/GJORM.2018.05.555660 Ovarian Ectopic Pregnancy with Newborn at Term: A Case Report Müller R1, Quirino I1* and Quintairos R2 1Fundação Santa Casa de Misericórdia do Pará, Brazil 2Hospital Porto Dias, Brazil Submission: June 05, 2018; Published: July 16, 2018 *Corresponding author: Quirino I, Fundação Santa Casa de Misericórdia do Pará, 1040,Rua Bernal do Couto. Belém, Brazil, Tel: Email: Keywords: Ovarian pregnancy; Ectopic pregnancy; Non-tubal ectopic pregnancy; Circulatory collapse; Wedge resection; Radical; Oophorectomy; Patients; Scotoma; Kidney; Epigastralgy; Ultrasonography; Hemorrhage; Thromboembolism; Urinary tract; Intrauterine growth; Placental; Lysis of adhesion; Ovarian gestation; Fetal vitality; Local vascularization Introduction ultrasound was single fetal in pelvic presentation, longitudinal Ovarian pregnancies represent between 1 to 6% of all situation, gestational age of 30 weeks and 4 days, estimated ectopic pregnancies and remain a challenge for the diagnosis [1], weight of 1528g. even with technological advancements in imaging methods. It is estimated that this event occurs in up to 1:70.000 pregnancies The patient was submitted to a complementary transvaginal and one of the consequences is the rupture at an earlier stage, US, where it was observed in right adnexal region uterus associated with high rates of circulatory collapse and maternal image, measuring 12cm in the largest diameter with 10mm -

XI. COMPLICATIONS of PREGNANCY, Childbffith and the PUERPERIUM 630 Hydatidiform Mole Trophoblastic Disease NOS Vesicular Mole Ex
XI. COMPLICATIONS OF PREGNANCY, CHILDBffiTH AND THE PUERPERIUM PREGNANCY WITH ABORTIVE OUTCOME (630-639) 630 Hydatidiform mole Trophoblastic disease NOS Vesicular mole Excludes: chorionepithelioma (181) 631 Other abnormal product of conception Blighted ovum Mole: NOS carneous fleshy Excludes: with mention of conditions in 630 (630) 632 Missed abortion Early fetal death with retention of dead fetus Retained products of conception, not following spontaneous or induced abortion or delivery Excludes: failed induced abortion (638) missed delivery (656.4) with abnormal product of conception (630, 631) 633 Ectopic pregnancy Includes: ruptured ectopic pregnancy 633.0 Abdominal pregnancy 633.1 Tubalpregnancy Fallopian pregnancy Rupture of (fallopian) tube due to pregnancy Tubal abortion 633.2 Ovarian pregnancy 633.8 Other ectopic pregnancy Pregnancy: Pregnancy: cervical intraligamentous combined mesometric cornual mural - 355- 356 TABULAR LIST 633.9 Unspecified The following fourth-digit subdivisions are for use with categories 634-638: .0 Complicated by genital tract and pelvic infection [any condition listed in 639.0] .1 Complicated by delayed or excessive haemorrhage [any condition listed in 639.1] .2 Complicated by damage to pelvic organs and tissues [any condi- tion listed in 639.2] .3 Complicated by renal failure [any condition listed in 639.3] .4 Complicated by metabolic disorder [any condition listed in 639.4] .5 Complicated by shock [any condition listed in 639.5] .6 Complicated by embolism [any condition listed in 639.6] .7 With other -

Hospital Acquired Conditions Diagnostic Codes and Descriptions
HOSPITAL ACQUIRED CONDITIONS DIAGNOSTIC CODES AND DESCRIPTIONS DX DX LONG 630 Hydatidiform mole 631.0 Inappropriate change in quantitative human chorionic gonadotropin (hCG) in early pregnancy 631.8 Other abnormal products of conception 632 Missed abortion 633.00 Abdominal pregnancy without intrauterine pregnancy 633.01 Abdominal pregnancy with intrauterine pregnancy 633.10 Tubal pregnancy without intrauterine pregnancy 633.11 Tubal pregnancy with intrauterine pregnancy 633.20 Ovarian pregnancy without intrauterine pregnancy 633.21 Ovarian pregnancy with intrauterine pregnancy 633.80 Other ectopic pregnancy without intrauterine pregnancy 633.81 Other ectopic pregnancy with intrauterine pregnancy 633.90 Unspecified ectopic pregnancy without intrauterine pregnancy 633.91 Unspecified ectopic pregnancy with intrauterine pregnancy 634.00 Spontaneous abortion, complicated by genital tract and pelvic infection, unspecified 634.01 Spontaneous abortion, complicated by genital tract and pelvic infection, incomplete 634.02 Spontaneous abortion, complicated by genital tract and pelvic infection, complete 634.10 Spontaneous abortion, complicated by delayed or excessive hemorrhage, unspecified 634.11 Spontaneous abortion, complicated by delayed or excessive hemorrhage, incomplete 634.12 Spontaneous abortion, complicated by delayed or excessive hemorrhage, complete 634.20 Spontaneous abortion, complicated by damage to pelvic organs or tissues, unspecified 634.21 Spontaneous abortion, complicated by damage to pelvic organs or tissues, incomplete 634.22 -

Ovarian Pregnancy Widayat1, Ariadi2 Affiliations : 1
Vol. 3, No. 2, Jul-Dec 2019 ANDALAS OBSTETRICS AND GYNECOLOGY JOURNAL Alamat Korespondensi: Ruang Redaksi Andalas Obstetrics and Gynecology Journal, Lantai 3 PPDS Obstetri dan Ginekologi Universitas Andalas, RSUP DR. M. Djamil Padang, Jl. Perintis Kemerdekaan Padang, Sumatera Barat 25127 Website: eISSN : 2579-8324 pISSN : 2579-8323 http://jurnalobgin.fk.unand.ac.id/index.php/JOE CASE REPORT Ovarian Pregnancy Widayat1, Ariadi2 Affiliations : 1. Resident of Obstetrics and Gynecology, Faculty of Medicine, Andalas University, Dr. M. Djamil Central General Hospital Padang; 2. Obstetrics and Gynecology Department, Faculty of Medicine, Andalas University, Dr. M. Djamil Central General Hospital Padang Correspondence: Widayat, email : [email protected], Hp: 082392042749 Abstract Objective: To report cases of ovarian pregnancy Materials and Methods: This article describes a case report of a 33 year old woman, with a diagnosis of Ovarian Pregnancy at 6-7 weeks gravid G2P0A1H0. The patient came to the emergency room Dr. M. Djamil Padang. The ultrasound examination gives the impression of an ectopic pregnancy in the right ampulla tube. After laparoscopy, an ectopic pregnancy was seen in the right ovary without bleeding. Right ovarian pregnancy impression. Partial Oophorectomy was performed and tissue evacuation with bleeding during the procedure ± 30 cc. Results: Patients receiving laparoscopic intervention showed an ectopic pregnancy in the right ovary without bleeding, the left ovary was within normal limits. Right ovarian pregnancy impression. Partial Oophorectomy was performed and tissue evacuation with bleeding during the procedure ± 30 cc. The tissue was examined for histology of anatomic pathology. Conclusion: Ovarian pregnancy is one of the rarest forms of ectopic pregnancy, it is sometimes difficult to diagnose because it can be confused with tubal ectopic pregnancy or hemorrhagic ovarian cyst. -

Ruptured Primary Ovarian Pregnancy: a Rare Case Report Shrestha A,1 Chawla CD,1 Shrestha RM2
KATHMANDU UNIVERSITY MEDICAL JOURNAL Ruptured Primary Ovarian Pregnancy: A Rare Case Report Shrestha A,1 Chawla CD,1 Shrestha RM2 1Department of Obstetrics and gynecology 2Department of Pathology Kathmandu University Hospital, Dhulikhel Hospital Kavre, Nepal ABSTRACT Ovarian pregnancy is an uncommon presentation of ectopic gestation and usually, it ends with rupture before the end of the first trimester. Its presentation often Corresponding Author is difficult to distinguish from that of tubal ectopic pregnancy and hemorrhagic Abha Shrestha ovarian cyst. We report a rare primary ruptured ovarian pregnancy in a 26 years lady. Department of Obstetrics and gynecology, Kathmandu University Hospital, Dhulikhel Hospital KEYWORDS Kavre, Nepal First trimester, hemorrhagic ovarian cyst, ovarian pregnancy E-mail: [email protected] Citation Shrestha A, Chawla CD, Shrestha RM. Ruptured Primary Ovarian Pregnancy: A Rare Case Report. Kathmandu Univ Med J 2012;39(3):76-77. INTRODUCTION Ovarian pregnancy is an uncommon presentation of ectopic in the right ovary of the MSD (mean sac diameter) of 15 mm gestation as per the criteria described by Speigelberg.1 corresponding to the sixth gestational week. Free fluid was Usually, it ends with rupture, which occurs before the also noted in the Douglas pouch. The Culdocentesis was end of the first trimester. Its presentation often is difficult positive for hemoperitoneum. So the diagnosis was made to distinguish from that of tubal ectopic pregnancy as ruptured ectopic pregnancy. Henceforth, emergency and hemorrhagic ovarian cyst. The prevalence is up to laparotomy was performed and found to be ruptured right approximately 1-3% of all extrauterine pregnancies.2 ovarian pregnancy as shown in fig 1. -

Ovarian Pregnancy - a Rare Case Report
CASE REPORT Ovarian Pregnancy - A Rare Case Report *SF Siddiqua1, S Abbasi2, S Rijvi3, AS Hasan4 ABSTRACT Ovarian pregnancy is a rare form of the non-tubal ectopic pregnancy. Primary ovarian ectopic pregnancy means implantation of the gestational sac in the ovary. Its incidence after natural conception ranges from 1 in 2000 to 1 in 60 000 deliveries and accounts for 3% of all ectopic pregnancies. It ends with rupture before the end of the first trimester. The preoperative diagnosis of this type of pregnancy is not easy. It is characterized by a poor clinical symptomatology and a difficult ultrasound diagnosis but confirmed by histological findings. For the management, Conservative laparoscopic surgery involves ovarian resection or aspiration of the pregnancy with coagulation of the implantation site. However, in case with profuse intraperitoneal bleeding an oophorectomy or salpingo-oophorectomy may be necessary to achieve hemostasis. We report here one such uncommon case of ovarian ectopic pregnancy. Our patient is a 27 years old nulliparous woman came with severe hypogastric pain. During laparoscopy, ruptured ovarian ectopic pregnancy was diagnosed, and wedge resection of the ovary was only done. Histopathological examination confirmed it to be an ovarian ectopic pregnancy. Key Words: Ectopic Pregnancy, Ovarian Pregnancy, Laparoscopy. Introduction An ectopic pregnancy is characterized by ovarian ligament, ovarian tissue is histologically implantation and development of an embryo outside distinguishable in the several means of the wall of the uterine cavity1. Ectopic pregnancies can around the pregnancy. These criteria are generally occur in the ovary (3.2%). The surgical criteria attributed to ovarian pregnancy.2 The ovary can remain hard to prove. -

Ovarian Ectopic Pregnancy: a 10 Years’ Experience and Review of Literature
Iran J Reprod Med Vol. 12. No. 12. pp: 825-830, December 2014 Original article Ovarian ectopic pregnancy: A 10 years’ experience and review of literature Lajya Devi Goyal1 M.D., Rimpy Tondon2 M.D., Poonam Goel2 M.D., Alka Sehgal2 M.D. 1. Department of Obstetrics and Abstract Gynecology, Guru Gobind Singh Medical College and Hospital, Background: Primary ovarian pregnancy is one of the rarest forms of ectopic Faridkot, Panjab, India. pregnancy having incidence of 1/7000-1/40,000 in live births and 0.5-3% of all 2. Department of Obstetrics and ectopic gestations. Intrauterine contraceptive device (IUCD), salpingitis, infertility, Gynecology, Govt. Medical and assisted reproductive techniques are the important risk factors. Approximately, College, Chandigarh, India. 75% terminate in first trimester and are often misdiagnosed as corpus luteum haemorrhage. Preoperative diagnosis by ultrasonography (USG) in early pregnancy can help in conservative medical/ surgical management. Objective: The aim of the present study was to find the incidence, risk factors, role of USG in pre-operative diagnosis, feasibility of conservative management with medical method or minimal invasive surgery in developing countries like India. Materials and Methods: We did a retrospective cross-sectional study of ovarian pregnancies managed at Government Medical College and Hospital Chandigarh between July 2000 to July 2010. We analyzed the incidence, risk factors, clinical presentation, management of ovarian pregnancy, and reviewed the literature. Results: Incidence of ovarian pregnancy was 4.9% of all ectopic pregnancies (14/523). Thirteen (93%) patients presented in first trimester with acute pain abdomen and of these ten patients had bleeding per vaginum. -

Primary Ovarian Pregnancy Case Report
Eastern Journal of Medicine 16 (2011) 277-280 M. A. Yüksel et al / Primary ovarian pregnancy Case Report Primary ovarian pregnancy: a report of four cases Mehmet Aytaç Yüksela, Remzi Abalıb, Fehmi Ünala, İlkbal Temela, Ahmet Birtan Borana aSB İstanbul Education and Research Hospital, Obstetrics and Gynecology Clinic, Istanbul, Turkey bNamık Kemal University, Obstetrics and Gynecology Department, Tekirdag, Turkey Abstract. Primary ovarian pregnancy is a rare form of ectopic pregnancy that constitutes 0.15-3 % of all ectopic pregnancies. Ovarian pregnancy results from the fertilization of trapped ovum within the follicle. Exact pre- operative diagnosis is often difficult, and with a few exceptions, the initial diagnosis is made on the operation table and the final diagnosis only through histopathology. The treatment of choice for ovarian pregnancy is usually ovarian wedge resection or oophorectomy, also there is a place for medical treatment of selected patients. Between October 2005 and May 2010, four cases of ovarian pregnancy that were detected and treated during laparotomy for suspected rupture of ectopic pregnancy are described. Three cases were treated by ovarian wedge resection. In one case, a single dose of 50 mg/m2 methotrexate treatment was given, but the same mentioned surgical procedure were performed due to rupture of gestation. In all cases diagnoses were confirmed by pathologic examination. We aimed to discuss a rare form of ectopic pregnancy, primary ovarian pregnancy, and highlight the diagnostic criteria Key words: Ovarian pregnancy, ectopic pregnancy abdominal pain. Abdominal examination of the 1. Introduction patient revealed lower quadrant defense and The incidence of ectopic pregnancy was sensitivity. During the vaginal examination, the reported to be between 1-2% and fallopian tube uterus and both adnexa were sensitive to was shown to be the most frequent location (1).