7/26/2016 Pennsylvania Sea Grant Final Report
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Species of Greatest Conservation Need Species Accounts
2 0 1 5 – 2 0 2 5 Species of Greatest Conservation Need Species Accounts Appendix 1.4C-Amphibians Amphibian Species of Greatest Conservation Need Maps: Physiographic Provinces and HUC Watersheds Species Accounts (Click species name below or bookmark to navigate to species account) AMPHIBIANS Eastern Hellbender Northern Ravine Salamander Mountain Chorus Frog Mudpuppy Eastern Mud Salamander Upland Chorus Frog Jefferson Salamander Eastern Spadefoot New Jersey Chorus Frog Blue-spotted Salamander Fowler’s Toad Western Chorus Frog Marbled Salamander Northern Cricket Frog Northern Leopard Frog Green Salamander Cope’s Gray Treefrog Southern Leopard Frog The following Physiographic Province and HUC Watershed maps are presented here for reference with conservation actions identified in the species accounts. Species account authors identified appropriate Physiographic Provinces or HUC Watershed (Level 4, 6, 8, 10, or statewide) for specific conservation actions to address identified threats. HUC watersheds used in this document were developed from the Watershed Boundary Dataset, a joint project of the U.S. Dept. of Agriculture-Natural Resources Conservation Service, the U.S. Geological Survey, and the Environmental Protection Agency. Physiographic Provinces Central Lowlands Appalachian Plateaus New England Ridge and Valley Piedmont Atlantic Coastal Plain Appalachian Plateaus Central Lowlands Piedmont Atlantic Coastal Plain New England Ridge and Valley 675| Appendix 1.4 Amphibians Lake Erie Pennsylvania HUC4 and HUC6 Watersheds Eastern Lake Erie -

2018 Pennsylvania Summary of Fishing Regulations and Laws PERMITS, MULTI-YEAR LICENSES, BUTTONS
2018PENNSYLVANIA FISHING SUMMARY Summary of Fishing Regulations and Laws 2018 Fishing License BUTTON WHAT’s NeW FOR 2018 l Addition to Panfish Enhancement Waters–page 15 l Changes to Misc. Regulations–page 16 l Changes to Stocked Trout Waters–pages 22-29 www.PaBestFishing.com Multi-Year Fishing Licenses–page 5 18 Southeastern Regular Opening Day 2 TROUT OPENERS Counties March 31 AND April 14 for Trout Statewide www.GoneFishingPa.com Use the following contacts for answers to your questions or better yet, go onlinePFBC to the LOCATION PFBC S/TABLE OF CONTENTS website (www.fishandboat.com) for a wealth of information about fishing and boating. THANK YOU FOR MORE INFORMATION: for the purchase STATE HEADQUARTERS CENTRE REGION OFFICE FISHING LICENSES: 1601 Elmerton Avenue 595 East Rolling Ridge Drive Phone: (877) 707-4085 of your fishing P.O. Box 67000 Bellefonte, PA 16823 Harrisburg, PA 17106-7000 Phone: (814) 359-5110 BOAT REGISTRATION/TITLING: license! Phone: (866) 262-8734 Phone: (717) 705-7800 Hours: 8:00 a.m. – 4:00 p.m. The mission of the Pennsylvania Hours: 8:00 a.m. – 4:00 p.m. Monday through Friday PUBLICATIONS: Fish and Boat Commission is to Monday through Friday BOATING SAFETY Phone: (717) 705-7835 protect, conserve, and enhance the PFBC WEBSITE: Commonwealth’s aquatic resources EDUCATION COURSES FOLLOW US: www.fishandboat.com Phone: (888) 723-4741 and provide fishing and boating www.fishandboat.com/socialmedia opportunities. REGION OFFICES: LAW ENFORCEMENT/EDUCATION Contents Contact Law Enforcement for information about regulations and fishing and boating opportunities. Contact Education for information about fishing and boating programs and boating safety education. -

West Branch Subbasin AMD Remediation Strategy
Publication 254 West Branch Susquehanna Subbasin May 2008 AMD Remediation Strategy: West Branch Susquehanna Background, Data Assessment River Task Force and Method Development Despite the enormous legacy ■ INTRODUCTION Pristine setting along the West Branch Susquehanna River. of pollution from abandoned mine The West Branch Susquehanna drainage (AMD) in the West Subbasin, draining a 6,978-square-mile Branch Susquehanna Subbasin, area in northcentral Pennsylvania, is the there has been mounting support largest of the six major subbasins in and enthusiasm for a fully restored the Susquehanna River Basin (Figure 1). watershed. Under the leadership The West Branch Susquehanna of Governor Edward G. Rendell Subbasin is one of extreme contrasts. While and with support from it has some of the Commonwealth’s Trout Unlimited, Pennsylvania most pristine and treasured waterways, Department of Environmental including 1,249 miles of Exceptional Protection Secretary Kathleen Value streams and scenic forestlands and mountains, it also unfortunately M. Smith McGinty established the West bears the legacy of past Branch Susquehanna River Task unregulated mining. With Abandoned mine lands in Clearfield County. Force (Task Force) in 2004. 1,205 miles of waterways The goal of the Task Force is to impaired by AMD, it is the assist and advise the department and most AMD-impaired region its partners as they work toward of the entire Susquehanna the long-term goal to remediate the River Basin (Figure 2). At its most degraded region’s AMD. sites, the West Branch The Task Force is comprised Susquehanna River contains of state, federal, and regional acidity concentrations of agencies, Trout Unlimited, and nearly 200 milligrams per other conservation and watershed liter (mg/l), and iron and aluminum concentrations of organizations (members are identified A. -

Lycoming County
LYCOMING COUNTY START BRIDGE SD MILES PROGRAM IMPROVEMENT TYPE TITLE DESCRIPTION COST PERIOD COUNT COUNT IMPROVED Bridge rehabilitation on State Route 2014 over Lycoming Creek in the City of BASE Bridge Rehabilitation State Route 2014 over Lycoming Creek Williamsport 1 $ 2,100,000 1 0 0 Bridge replacement on PA 973 over the First Fork of Larry's Creek in Mifflin BASE Bridge Replacement PA 973 over the First Fork of Larry's Creek Township and epoxy overlay on PA 973 over Larry's Creek in Mifflin Township 1 $ 1,577,634 2 1 0 BASE Bridge Rehabilitation State Route 2039 over Mill Creek Bridge replacement on State Route 2039 over Mill Creek in Loyalsock Township 1 $ 398,640 1 1 0 Bridge rehabilitation on Township Road 434 over Mosquito Creek in Armstrong BASE Bridge Rehabilitation Township Road 434 over Mosquito Creek Township 3 $ 1,220,000 1 1 0 Bridge truss rehabilitation on State Route 2069 over Little Muncy Creek in BASE Bridge Rehabilitation State Route 2069 over Little Muncy Creek Moreland Township 1 $ 1,000,000 1 1 0 Bridge replacement on PA 87 over Tributary to Loyalsock Creek in Upper Fairfield BASE Bridge Replacement PA 87 over Tributary to Loyalsock Creek Township 3 $ 1,130,000 1 1 0 Bridge replacement on State Route 2001 (Elimsport Road) over Branch of Spring BASE Bridge Replacement State Route 2001 over Branch of Spring Creek #1 Creek in Washington Township 1 $ 1,270,000 1 1 0 BASE Bridge Replacement PA 414 over Upper Pine Bottom Run Bridge replacement on PA 414 over Upper Pine Bottom Run in Cummings Township 2 $ 1,620,000 1 1 -

Public Votes Loyalsock As PA River of the Year Perkiomen TU Leads
Winter 2018 Publication of the Pa. Council of Trout Unlimited www.patrout.org Perkiomen TU Students to leads restoration research brookies project on on Route 6 trek By Charlie Charlesworth namesake creek PATU President By Thomas W. Smith Perkiomen Valley TU President In summer 2018, six college students from our PATU 5 Rivers clubs will spend a The Perkiomen Valley Chapter of month trekking across Pennsylvania’s U.S. Trout Unlimited partnered with Sundance Route 6. Their purpose will be to explore, Creek Consulting, the Montgomery do research, collect data and still have time County Conservation District, Penn State do a little bit of fishing in the northern tier’s Master Watershed Stewards and Upper famed brook trout breeding grounds. Perkiomen High School for a stream They will be supported by the PA Fish restoration project on Perkiomen Creek, and Boat Commission, three colleges in- Contributed Photo which was carried out over five days in Volunteers work on a stream restora- cluding Mansfield, Keystone and hopefully See CREEK, page 7 tion project along Perkiomen Creek. See TREK, page 2 Public votes Loyalsock as PA River of the Year By Pennsylvania DCNR Home to legions of paddlers, anglers, and other outdoors enthusiasts in north central Pennsylvania, Loyalsock Creek has been voted the 2018 Pennsylvania River of the Year. The public was invited to vote online, choosing from among five waterways nominated across the state. Results were pariveroftheyear.org Photo See RIVER, page 2 Loyalsock Creek was voted 2018 Pennsylvania River of the Year. IN THIS ISSUE Keystone Coldwater Conference ..........................3 How to become a stream advocate.......................6 Headwaters .............................................................4 Minutes ....................................................................8 Treasurer’s Notes ...................................................5 Chapter Reports .................................................. -

Wild Trout Waters (Natural Reproduction) - September 2021
Pennsylvania Wild Trout Waters (Natural Reproduction) - September 2021 Length County of Mouth Water Trib To Wild Trout Limits Lower Limit Lat Lower Limit Lon (miles) Adams Birch Run Long Pine Run Reservoir Headwaters to Mouth 39.950279 -77.444443 3.82 Adams Hayes Run East Branch Antietam Creek Headwaters to Mouth 39.815808 -77.458243 2.18 Adams Hosack Run Conococheague Creek Headwaters to Mouth 39.914780 -77.467522 2.90 Adams Knob Run Birch Run Headwaters to Mouth 39.950970 -77.444183 1.82 Adams Latimore Creek Bermudian Creek Headwaters to Mouth 40.003613 -77.061386 7.00 Adams Little Marsh Creek Marsh Creek Headwaters dnst to T-315 39.842220 -77.372780 3.80 Adams Long Pine Run Conococheague Creek Headwaters to Long Pine Run Reservoir 39.942501 -77.455559 2.13 Adams Marsh Creek Out of State Headwaters dnst to SR0030 39.853802 -77.288300 11.12 Adams McDowells Run Carbaugh Run Headwaters to Mouth 39.876610 -77.448990 1.03 Adams Opossum Creek Conewago Creek Headwaters to Mouth 39.931667 -77.185555 12.10 Adams Stillhouse Run Conococheague Creek Headwaters to Mouth 39.915470 -77.467575 1.28 Adams Toms Creek Out of State Headwaters to Miney Branch 39.736532 -77.369041 8.95 Adams UNT to Little Marsh Creek (RM 4.86) Little Marsh Creek Headwaters to Orchard Road 39.876125 -77.384117 1.31 Allegheny Allegheny River Ohio River Headwater dnst to conf Reed Run 41.751389 -78.107498 21.80 Allegheny Kilbuck Run Ohio River Headwaters to UNT at RM 1.25 40.516388 -80.131668 5.17 Allegheny Little Sewickley Creek Ohio River Headwaters to Mouth 40.554253 -80.206802 -
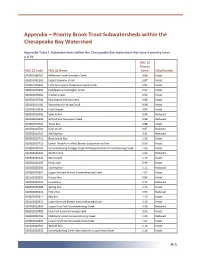
Appendix – Priority Brook Trout Subwatersheds Within the Chesapeake Bay Watershed
Appendix – Priority Brook Trout Subwatersheds within the Chesapeake Bay Watershed Appendix Table I. Subwatersheds within the Chesapeake Bay watershed that have a priority score ≥ 0.79. HUC 12 Priority HUC 12 Code HUC 12 Name Score Classification 020501060202 Millstone Creek-Schrader Creek 0.86 Intact 020501061302 Upper Bowman Creek 0.87 Intact 020501070401 Little Nescopeck Creek-Nescopeck Creek 0.83 Intact 020501070501 Headwaters Huntington Creek 0.97 Intact 020501070502 Kitchen Creek 0.92 Intact 020501070701 East Branch Fishing Creek 0.86 Intact 020501070702 West Branch Fishing Creek 0.98 Intact 020502010504 Cold Stream 0.89 Intact 020502010505 Sixmile Run 0.94 Reduced 020502010602 Gifford Run-Mosquito Creek 0.88 Reduced 020502010702 Trout Run 0.88 Intact 020502010704 Deer Creek 0.87 Reduced 020502010710 Sterling Run 0.91 Reduced 020502010711 Birch Island Run 1.24 Intact 020502010712 Lower Three Runs-West Branch Susquehanna River 0.99 Intact 020502020102 Sinnemahoning Portage Creek-Driftwood Branch Sinnemahoning Creek 1.03 Intact 020502020203 North Creek 1.06 Reduced 020502020204 West Creek 1.19 Intact 020502020205 Hunts Run 0.99 Intact 020502020206 Sterling Run 1.15 Reduced 020502020301 Upper Bennett Branch Sinnemahoning Creek 1.07 Intact 020502020302 Kersey Run 0.84 Intact 020502020303 Laurel Run 0.93 Reduced 020502020306 Spring Run 1.13 Intact 020502020310 Hicks Run 0.94 Reduced 020502020311 Mix Run 1.19 Intact 020502020312 Lower Bennett Branch Sinnemahoning Creek 1.13 Intact 020502020403 Upper First Fork Sinnemahoning Creek 0.96 -

A Town in History 1
Montoursville: a town in history 1 a town in history Contents …a snapshot in time of Montoursville history Chapter 1, Aboriginal Culture & Founding……………………………… 2 Chapter 2, Surviving the Wars…………………………………………………. 2 Chapter 3, Early Life………………………………………………………………… 3 Chapter 4, Lumber Built the Town………………………………………….. 4 Chapter 5, Rafting on the Loyalsock……………………………………….. 5 Chapter 6, Land Among the Waters Gave Us Recreation………… 5 Chapter 7, The Borough Waterworks………………………………………. 7 Chapter 8, The Water System Made Progress Possible…………… 8 Chapter 9, Schooling in Andrew’s Town………………………………….. 8 Chapter 10, Transportation……………………………………………………… 10 Chapter 11, Two Great Wars……………………………………………………. 11 Chapter 12, Visions from the Last Fifty Years………………………… 11 2 Historic Broad Street 8 Loyalsock Ave. Normal School 3 Home of Thomas Lloyd 9 First high school 3 Home of John Else 9 Montour Street School 4 Home of Gov. Shulze 9 Today’s high school 5 Park coaster 10 The “Green” bridge 5 Park Theatre 10 Old bridges 6 Indian Park today 10 Old view of the airport 6 Covered bridge 10 The airport today 7 John Hazel 12 Flight 800 memorial statue 8 Old borough building 14 Broad Street today Note: Pictures are marked as modern or historic There is a running timeline featuring key years in Montoursville history beginning on page 2 and continuing through page 11. This informational pamphlet and associated website created by Chris Garneau. Please see last page for more information. 2 Montoursville: a town in history 1. ABORIGINAL CULTURE to inhabit the land. The Susquehanna approximately 22 cents an acre. was a mecca for fisherman from Andrew Montour was twice married It was on February 19, 1850 that an act southeastern Pennsylvania and and fathered two sons, Nicholas and of the General Assembly of Delaware. -

EPCAMR 2007 2Nd Quarter Board Meeting Watershed & Cooperating Organization Reports – April 26, 2007 Presidents Report Ed W
EPCAMR 2007 2nd Quarter Board Meeting Watershed & Cooperating Organization Reports – April 26, 2007 Presidents Report Ed Wytovich, President • Attended a Confluence Summit on Stormwater • Coordinating a site visit with Kelly Donaldson working with the Chesapeake Bay Foundation who is producing another film documentary this summer on AMD and AML Reclamation. They plan to visit Schuylkill next Friday. Wiconisco Creek Restoration Association • Central PA Conservancy acquired of 50 acres along the Wiconisco Creek with DCNR Funding (50% match grant) and WCRA to develop into State Game Lands and rail trailhead access. • Bear Creek Project Phase Dauphin County Commissioners committed $610 K toward the project putting in for a GG for $300 K for the rest. PACD M. Irvil Kear, Chair Robert Hughes, Regional Coordinator Michael Hewitt, Watershed Coordinator • Took over Ron Rohall’s position and continues his work with the AML Campaign. Sat in on a conference call talking about their stand on Title 4 Rulemaking. They feel there is a need for transparency and making sure the dollars are spent correctly. They are pushing for the full 30% setaside for mine drainage work and operation and maintenance work. It may take up to 2022 for all Priority 1 & 2 projects to be done, to be “certified.” • Pennsylvania has the most Priority 1 & 2 sites of all states. West Virginia is #2. West Virginia may be placing their money into water supply restoration. Columbia County Cathy Haffner, Watershed Specialist Maryruth Wagner, District Manager Catawissa Creek Restoration Association • A Rivers Conservation Plan for the Catawissa Creek watershed is in its initial stages. -

Ghost Towns of North Mountain: Ricketts, Mountain Springs, Stull
G HOST T OWNS OF NORTH MOUNTAIN: RICKETTS, MOUNTAIN SPRINGS AND STULL F. Charles Petrillo 1991 Introduction he rural and mountainous area surrounding Ricketts Glen State Harvey’s Lake, and at Stull (1891-1906) on Bowman’s Creek, and for Park, at the intersection of Luzerne, Wyoming, and Sullivan coun- large lumbering operations in the towns of Lopez (1887-1905) on Tties, is known as North Mountain. The mountain range forms a Loyalsock Creek, Jamison City (1889-1912) on Fishing Creek, and at watershed between the north and west branches of the Susquehanna Ricketts (1890-1913) on Mehoopany Creek. River. At Ricketts Glen, Bowman’s Creek begins to flow generally east- Ice-cutting was another North Mountain industry during this era, ward through the now deserted ice-cutting town of Mountain Springs, with its major center at Mountain Springs (1891-1948) along along the former lumbering town of Stull, beyond the old tannery town Bowman’s Creek, and to a smaller extent at Lake Ganoga (1896- of Noxen, into the farming valley of Beaumont, and onward to the c.1915), a private lake development near the state park. The ice indus- Susquehanna River below Tunkhannock. North of Ricketts Glen, try continued to operate for another three decades after the end of lum- Mehoopany Creek flows northeasterly through the ghost lumber town of bering in North Mountain, closing as mechanical refrigeration came Ricketts, eventually flowing into the Susquehanna River at the town of into general household use immediately after World War II. Mehoopany, another old lumbering center. In central Sullivan County, Loyalsock Creek descends from World’s The Lumber Industry End State Park and passes through Lopez, once the county’s major lum- bering center. -

PA Wilds Fishing Guide
Allegheny National Forest Get Your Fishing License & Go WILDS! Ranger Stations Pennsylvania offers a variety of tourist and resident license options – from a one-day Bradford: 814-362-4613 PA Game Commission permit to a lifetime license – so there’s one that’s just right for you. And getting your Marienville: 814-927-6628 license is quick and easy. Licenses can be purchased and printed from the convenience of : 814-723-5150 Serving the following PA Wilds Counties: your own home. Simply visit and click on the “Get Your Fishing Warren www.fishandboat.com Cameron, Clearfield, License” link on the home page or purchase your license at one of 1200 issuing agents Clinton, Elk, Lycoming, statewide. Anglers 16 years of age and older must possess a valid fishing license to fish fs.usda.gov/allegheny McKean, Potter, Tioga in Pennsylvania.CAMERON, CLARION, ELK, FOREST & JEFFERSON MCKEAN COUNTY 1566 South Route 44 Highway COUNTIES Allegheny National Forest PA Fish & Boat Commission P.O. Box 5038 PickNorthwest the licensePennsylvania’s that best fits you.Visitors Bureau Jersey Shore, PA 17740 Great Outdoors 80 East Corydon Street, Serving the following PA Wilds counties: (570) 398-4744 Visitors Bureau Suite 114 TYPE OF LICENSE Bradford,AGE PA 16701 COST Cameron, Clearfield, 2801 Maplevale Road Serving the following PA Wilds Counties: ResidentBrookville, PA 15825 800-473-937016-64 $22.00 Clinton, Elk, Jefferson, Clarion, Forest, Senior 814-849-5197Resident (Annual) www.visitANF.com65 & up $11.00 FishingLycoming, in the McKean, Pennsylvania Wilds .....................................1 www.visitpago.com Potter, Tioga Jefferson, Warren Senior Resident (Lifetime) 65 & up $51.00 P.O. -

Lycoming County Chesapeake Bay Tributary Strategy
Lycoming County’s Implementation Plan For the Chesapeake Bay Tributary Strategy The Lycoming County Conservation District’s Board of Directors approved this version of the Lycoming County Implementation Plan for the Chesapeake Bay Tributary Strategy during their February18, 2015 meeting. 1 Table of Contents County Description……………………………………………………………………………….Page 3 Past Accomplishments Impaired Waters of Lycoming County…………………………………………….......................Page 5 US EPA Priority Agricultural Streams Impaired by High Total Nitrogen……………...………..Page 8 USDA-NRCS Priority Watersheds Priority Areas Map of Lycoming County’s Impaired Water…………………………………………………...Page 10 Technical Resource……………………………………………………………….......................Page 11 Funding Source Best Management Practices Agricultural Compliance…...…………………………………………………………………...Page 12 Agricultural Land Preservation Programs and Long Term Easement Programs Barnyard Runoff Control………………………………………………………….......................Page13 Conservation Plans Cover Crops……………………………………………………………………………………..Page 14 Dirt and Gravel Road Pollution Prevention Program Managed Precision Agriculture No-till Farming Nutrient Management Planning………………………………………………………………....Page 15 Nutrient Trading…………………………………………………...……………………………Page 16 Public Education Stream Bank Fencing, Off Stream Watering Systems and Riparian Forest Buffers……………Page 17 Stream Bank Stabilization and Stream Bank Restoration………………………........................Page 18 Storm Water Management…………………………………………...………………………….Page 19 Urban Nutrient Management