Design and Synthesis of Multifunctional Polyesters With
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Attachment Properties of Blue Mussel (Mytilus Edulis L.) Byssus Threads on Culture-Based Artificial Collector Substrates
Aquacultural Engineering 42 (2010) 128–139 View metadata, citation and similar papers at core.ac.uk brought to you by CORE Contents lists available at ScienceDirect provided by Electronic Publication Information Center Aquacultural Engineering journal homepage: www.elsevier.com/locate/aqua-online Attachment properties of blue mussel (Mytilus edulis L.) byssus threads on culture-based artificial collector substrates M. Brenner a,b,c,∗, B.H. Buck a,c,d a Alfred Wegener Institute for Polar and Marine Research (AWI), Am Handelshafen 12, 27570 Bremerhaven, Germany b Jacobs University Bremen, Campus Ring 1, 28759 Bremen, Germany c Institute for Marine Resourses (IMARE), Klußmannstraße 1, 27570 Bremerhaven, Germany d University of Applied Sciences Bremerhaven, An der Karlstadt 8, 27568 Bremerhaven, Germany article info abstract Article history: The attachment strength of blue mussels (Mytilus edulis) growing under exposed conditions on 10 differ- Received 25 May 2009 ent artificial substrates was measured while assessing microstructure of the applied substrate materials. Accepted 9 February 2010 Fleece-like microstructure attracted especially mussel larvae, however, most settled individuals lost attachment on this type of microstructure with increasing size during the time of experiment. Sub- Keywords: strates with thick filaments and long and fixed appendices were less attractive to larvae but provided a Spat collectors better foothold for juvenile mussels as shown by the results of the dislodgement trials. In addition these Offshore aquaculture appendices of substrates could interweave with the mussels, building up a resistant mussel/substrate con- Offshore wind farms Mytilus edulis glomerate. Our results show that a mussel byssus apparatus can withstand harsh conditions, if suitable Dislodgement substrates are deployed. -

The Peculiar Protein Ultrastructure of Fan Shell and Pearl Oyster Byssus
Soft Matter View Article Online PAPER View Journal | View Issue A new twist on sea silk: the peculiar protein ultrastructure of fan shell and pearl oyster byssus† Cite this: Soft Matter, 2018, 14,5654 a a b b Delphine Pasche, * Nils Horbelt, Fre´de´ric Marin, Se´bastien Motreuil, a c d Elena Macı´as-Sa´nchez, Giuseppe Falini, Dong Soo Hwang, Peter Fratzl *a and Matthew James Harrington *ae Numerous mussel species produce byssal threads – tough proteinaceous fibers, which anchor mussels in aquatic habitats. Byssal threads from Mytilus species, which are comprised of modified collagen proteins – have become a veritable archetype for bio-inspired polymers due to their self-healing properties. However, threads from different species are comparatively much less understood. In particular, the byssus of Pinna nobilis comprises thousands of fine fibers utilized by humans for millennia to fashion lightweight golden fabrics known as sea silk. P. nobilis is very different from Mytilus from an ecological, morphological and evolutionary point of view and it stands to reason that the structure– Creative Commons Attribution 3.0 Unported Licence. function relationships of its byssus are distinct. Here, we performed compositional analysis, X-ray diffraction (XRD) and transmission electron microscopy (TEM) to investigate byssal threads of P. nobilis, as well as a closely related bivalve species (Atrina pectinata) and a distantly related one (Pinctada fucata). Received 20th April 2018, This comparative investigation revealed that all three threads share a similar molecular superstructure Accepted 18th June 2018 comprised of globular proteins organized helically into nanofibrils, which is completely distinct from DOI: 10.1039/c8sm00821c the Mytilus thread ultrastructure, and more akin to the supramolecular organization of bacterial pili and F-actin. -

Extensible Byssus of Pinctada Fucata
www.nature.com/scientificreports OPEN Extensible byssus of Pinctada fucata: Ca2+-stabilized nanocavities and a thrombospondin-1 protein Received: 18 June 2015 1,2 1 1 1 1 1 Accepted: 15 September 2015 Chuang Liu , Shiguo Li , Jingliang Huang , Yangjia Liu , Ganchu Jia , Liping Xie & 1 Published: 08 October 2015 Rongqing Zhang The extensible byssus is produced by the foot of bivalve animals, including the pearl oyster Pinctada fucata, and enables them to attach to hard underwater surfaces. However, the mechanism of their extensibility is not well understood. To understand this mechanism, we analyzed the ultrastructure, composition and mechanical properties of the P. fucata byssus using electron microscopy, elemental analysis, proteomics and mechanical testing. In contrast to the microstructures of Mytilus sp. byssus, the P. fucata byssus has an exterior cuticle without granules and an inner core with nanocavities. The removal of Ca2+ by ethylenediaminetetraacetic acid (EDTA) treatment expands the nanocavities and reduces the extensibility of the byssus, which is accompanied by a decrease in the β-sheet conformation of byssal proteins. Through proteomic methods, several proteins with antioxidant and anti-corrosive properties were identified as the main components of the distal byssus regions. Specifically, a protein containing thrombospondin-1 (TSP-1), which is highly expressed in the foot, is hypothesized to be responsible for byssus extensibility. Together, our findings demonstrate the importance of inorganic ions and multiple proteins for bivalve byssus extension, which could guide the future design of biomaterials for use in seawater. In recent years, marine animals have attracted considerable attention in various areas of bioinspired engineering1–4. -

The Effect of Various Spat Collector Materials to Spat Attachment
Journal of Coastal Development ISSN : 1410-5217 Volume 15, Number 1, October 2011 : 34 - 44 Accredited : 83/Dikti/Kep/2009 Original Paper THE EFFECT OF VARIOUS SPAT COLLECTOR MATERIALS FOR SPAT ATTACHMENT OF PEARL OYSTER (Pinctada maxima) Endang Arini1 and Nur Taufiq Syamsudin Putra Jaya2 1Fisheries Department, Faculty of Fisheries and Marine Science, Diponegoro University 2Marine Science Department Faculty of Fisheries and Marine Science, Diponegoro University Jl. Prof. Soedarto, SH, Tembalang Campus, Semarang -50275, Indonesia Received : April, 20, 2011 ; Accepted : June, 30, 2011 ABSTRACT Early stage development of pearl oyster spat was very critical, hence spat required suitable substrates for their settlement to complete their metamorphosis. In this stage, byssus for the attachment is vulnerable and easy to break if there are some disturbance e.g. water movement, hence spat easily falling onto the bottom layer which may have various dirt materials leading to the dead of the spat. In the pearl oyster culture spat collector is important. The aim of this study is to study the effect of different collector materials to the number of spat settled, to reveal the best collector materials and the endurance of spat at the collector. This study was conducted in PT Autore Pearl Culture, at Sumbawa island, Nusa Tenggara Province. Materials used in this research were collectors of different substance i.e. asbestos (A), bamboo (B), polyethylene and roof ceramic (D) substance. The method used was laboratories experiment with Completely Random Design by 4 treatments and 3 replications. Data taken were the number of spat settlement, endurance test, and water quality. Data tested with Analysis of Variance (ANOVA) and further tested with Duncan Duplicate Regional test. -

Unit � Chemistry of Textiles: Animal Fibres File:///C:/WWW/Courses/CHEM2402/Textiles/Animal Fibres.Html
Unit - Chemistry of Textiles: Animal Fibres file:///C:/WWW/Courses/CHEM2402/Textiles/Animal_Fibres.html Unit - Chemistry of Garments: Animal Fibres Alpaca , Angora , Byssus , Camel hair , Cashmere , Catgut , Chiengora , Guanaco , Llama , Mohair Pashmina , Qiviut , Rabbit , Silk , Sinew , Spider silk , Wool , Vicuña , Yak Silk Silk can be obtained from the cocoons of several types of caterpillar or silkworm, but it is now the Chinese silk moth (mulberry silkworm Bombyx mori ) reared in captivity that is mainly cultivated. See the Zanzibar Tribal Art web site for an explanation and diagram of the life cycle of the various silkworms . YouTube video clip of Silk factory in Beijing Natural silk is one of the strongest textile fibres, which are accounted for by the stretched-out molecular form. Silk (78% protein) is much stiffer than wool in spite of both being proteins made from amino acids chains. Silk fibres have fine draping qualities and are naturally crease-resistant and bring about a warm feel to the skin. Of the 3-4000 metres of fibre in a cocoon, laid out as a figure of eight by the movement of the head of the pupae, less that one third is generally usable with much of the remainder being processed separately. Silk emitted by the silkworm consists of two main proteins, sericin and fibroin, fibroin being the structural center of the silk, and serecin being the sticky material surrounding it. Fibroin is largely made up of the amino acids Gly-Ser-Gly-Ala-Gly-Ala and forms beta pleated sheets, β-keratin. 1 of 9 20/10/2011 08:28 PM Unit - Chemistry of Textiles: Animal Fibres file:///C:/WWW/Courses/CHEM2402/Textiles/Animal_Fibres.html R = H, glycine; R = CH , alanine; R = CH OH, serine 3 2 Hydrogen bonds form between chains, and side chains form above and below the plane of the hydrogen bond network. -

All Abstracts
European Cells and Materials Vol. 26. Suppl. 6, 2013 (page 1) ISSN 1473-2262 Sea-urchin inspired wet bioadhesives - a multidisciplinary quest towards biomimicry R Santos Biomedical and Oral Sciences Research Unit (UICOB), School of Dentistry of the University of Lisbon, Portugal INTRODUCTION: Adhesive mechanisms are underwater environment. More recently, beginning to be deciphered in some marine techniques such as proteomics and mass organisms that attach permanently with a cement spectrometry, demonstrated that tube feet are (mussels, barnacles) or use a viscous film to move unique mechano-sensory adhesive organs and and adhere (limpets). However, this is far from highlighted putative adhesive proteins. Work is in being true for sea urchins, that attach strongly but progress to identify the genes coding for these temporarily to the substratum. Sea urchins are adhesive proteins in order to produce recombinant exclusively benthic animals and to withstand analogues or partial adhesive motifs that can be hydrodynamic forces rely on hundreds of used to design new dental bioadhesives inspired on independent adhesive organs, their adoral tube millions of years of optimization through feet, specialized in locomotion and anchoring. biological selection. Tube feet consist of two functional units: a disc, which makes contact with the substratum, and a stem, which connects the disc to the test. Therefore, the tenacity with which an individual can anchor to a surface is determined by the strength of its numerous tube feet which, in turn, Fig. 1: Images of sea urchin tube feet attaching depends on the tensile strength of the stem and the underwater to the surface of a rock (A) and to adhesive power of the disc. -
![APPENDIX 1 [Available As a Download] List of Animal-Derived Fibres and Materials*](https://docslib.b-cdn.net/cover/4059/appendix-1-available-as-a-download-list-of-animal-derived-fibres-and-materials-3014059.webp)
APPENDIX 1 [Available As a Download] List of Animal-Derived Fibres and Materials*
APPENDIX 1 [available as a download] List of animal-derived fibres and materials*: Animal Materials & Fibres Colourings & Dyes Glues & Waxes Leather, Suede Wool / Animal hair Shell Ingredients derived Milk or egg protein Waxes from To note: Shearling including but Alpaca Capiz (mollusc) from insects Albumen animals wax is made by either not limited to: Angora Chitosan (crustacean) Carmine / Casein paint (milk Arachidyl bees or secretion of Exotic skins such as Boar bristles Nacre / Mother of Cochineal protein) propionate (similar the sebaceous glands Alligator Camel hair pearl Kermesic acid Casein glue (milk to lanolin) of wool-bearing Sheep / Lambskin Cashgora Pearl Lac protein) Beeswax animals - sometimes Goat Cashmere Sea shell Shellac Chinese Wax found in fabric Pig Guanaco (llama) Tekhelet (snail To note: (insects) softeners or water Cow Horsehair Horn / Bone glands) Urea (Urine from Lanolin / wax from resistant finishes Calfskin Huarizo (alpaca) Horn Tyrian purple (sea mammals) can be wool Buffalo Karakul (sheep) Bone Char snails) used to dissolve Shellac Wax To note: Catgut (type of cord Kemp (sheep) Ivory dyes Spermaceti Gelatin and Isinglass made from walls of Llama Inks from animals are Fur Glues from animals sheep / goat intestines) Merino Sepia sometimes used to Chamois Mohair Any animal fur Octopus ink Bone glue thicken adhesives, Pashmina Beaver or Urea Fish glue made from animal Silks Pantholops Otter fur Hide glue bones or fish Anaphe (moth) (antelope) Rabbit skin glue Antheraea (moth) Qiviut (muskox) Feather / down Gelatin Byssus (sea silk made Rabbit Hair Feather Isinglass from mollusc) Shahtoosh (antelope) Down Doupioni Suri (alpaca) Eri Vicuna (llama) Honan Yak Milk/casein fibre Muga Sea Silk Tussah Silk * This list was compiled December 2019 to be non-exhaustive and can be updated to reflect change in trends. -

Perna Viridis) Using Longline Culture and an Economic Comparison with Stake Culture on the North Coast of Java, Indonesia
Received: 26 February 2020 | Revised: 21 August 2020 | Accepted: 10 September 2020 DOI: 10.1111/are.14900 ORIGINAL ARTICLE Increased production of green mussels (Perna viridis) using longline culture and an economic comparison with stake culture on the north coast of Java, Indonesia Sri Rejeki1 | Adolphe O. Debrot2,3 | Anneke M. van den Brink2 | Restiana W. Ariyati1 | Lestari Lakshmi Widowati1 1Aquaculture Department, Faculty of Fisheries and Marine Sciences, Diponegoro Abstract University, Jl. Prof. H. Soedarto S.H, The effectiveness of green mussel (Perna viridis) cultivation was compared between Tembalang, Semarang, Indonesia longline culture and the traditional bamboo stake method using different mussel den- 2Wageningen Marine Research, Wageningen University and Research, IJmuiden, the sities in a rural area on the north coast of Java, Indonesia. The study took place in Netherlands a rural area about 2 km north-east from the city of Semarang where unsustainable 3Marine and Animal Ecology group, Department of Animal Sciences, shrimp and fish pond culture in the past has led to massive mangrove loss, spiralling Wageningen University, Wageningen, the environmental degradation and socio-economic disintegration. Mangrove-friendly Netherlands alternatives for a sustainable socio-economic recovery of local livelihoods are ur- Correspondence gently being sought. Longlines were more successful than the stake method in spat Adolphe O. Debrot, Wageningen Marine Research, Wageningen University and collection. Longlines also showed a small but significantly higher relative weight gain Research, Postal address: P.O Box 68, 1970 and specific growth rate for mussels than bamboo stakes. Mussels in lower densities AB IJmuiden, the Netherlands. Email: [email protected] showed higher survival and grew to larger individual sizes, but initial seeding density had no significant effect on relative weight gain or specific growth rate per stock- Funding information Netherlands Ministry of Agriculture, Nature ing. -

Self-Healing Silk from the Sea: Role of Helical Hierarchical Structure in Pinna Nobilis Byssus Mechanics
Self-healing silk from the sea: role of helical hierarchical structure in Pinna nobilis byssus mechanics. Delphine Pasche, Nils Horbelt, Frédéric Marin, Sébastien Motreuil, Peter Fratzl, Matthew J. Harrington To cite this version: Delphine Pasche, Nils Horbelt, Frédéric Marin, Sébastien Motreuil, Peter Fratzl, et al.. Self-healing silk from the sea: role of helical hierarchical structure in Pinna nobilis byssus mechanics.. Soft Matter, Royal Society of Chemistry, 2019, 15 (47), pp.9654-9664. 10.1039/c9sm01830a. hal-02404937 HAL Id: hal-02404937 https://hal.archives-ouvertes.fr/hal-02404937 Submitted on 6 Jan 2021 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Soft Matter View Article Online PAPER View Journal | View Issue Self-healing silk from the sea: role of helical hierarchical structure in Pinna nobilis Cite this: Soft Matter, 2019, 15,9654 byssus mechanics a a b b Delphine Pasche, Nils Horbelt, Fre´de´ric Marin, Se´bastien Motreuil, Peter Fratzla and Matthew J. Harrington *ac The byssus fibers of Mytilus mussel species have become an important role model in bioinspired materials research due to their impressive properties (e.g. high toughness, self-healing); however, Mytilids represent only a small subset of all byssus-producing bivalves. -

Zebra Mussels Anchor Byssal Threads Faster and Tighter Than Quagga Mussels in Flow
2027 The Journal of Experimental Biology 212, 2027-2036 Published by The Company of Biologists 2009 doi:10.1242/jeb.028688 Zebra mussels anchor byssal threads faster and tighter than quagga mussels in flow Suzanne M. Peyer1,*, Alice J. McCarthy1 and Carol Eunmi Lee1,2 1Department of Zoology and 2Center of Rapid Evolution (CORE), University of Wisconsin, Madison, WI 53706, USA *Author for correspondence (e-mail: [email protected]) Accepted 23 December 2008 SUMMARY While the invasive zebra mussel Dreissena polymorpha has rapidly spread throughout the Great Lakes and inland waterways, it is being displaced by the quagga mussel Dreissena bugensis in shallow water habitats. However, zebra mussels remain dominant in areas with higher water velocity. We hypothesized that the persistence of zebra over quagga mussels in habitats with higher water velocity might result from greater rate and strength of byssal thread attachment. We examined whether zebra mussels relative to quagga mussels have: (1) higher byssal thread synthesis rate, (2) lower dislodgment in flow and (3) greater mechanical force required for detachment from substrate. Specifically, we examined byssal thread synthesis rate and dislodgment of both species in response to water velocities of 0, 50, 100 and 180 cm s–1. Byssal thread synthesis rate was significantly higher for zebra than for quagga mussels at all velocities. Dislodgment from the substrate increased for both species with increasing velocity but was significantly lower for zebra than for quagga mussels. We also tested the mechanical force to detach mussels after short (32 h) and long (two and three months) periods of attachment on hard substrate. -
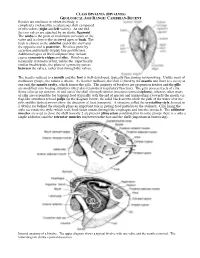
Bivales Are Molluscs in Which the Body Is Completely Enclosed by a Calcareous Shell Composed of Two Valves (Right and Left Valves)
CLASS BIVALVIA (BIVALVES) GEOLOGICAL AGE RANGE: CAMBRIAN-RECENT Bivales are molluscs in which the body is completely enclosed by a calcareous shell composed of two valves (right and left valves). At one end, the two valves are attached by an elastic ligament. The umbo is the point of maximum curvature of the valve and is close to the incurved apex or beak. The beak is closest to the anterior end of the shell and the opposite end is posterior. Bivalves grow by accretion and usually display fine growth lines. Additional types of shell sculpture may include coarse concentric ridges and ribs. Bivalves are bilaterally symmetrical but, unlike the superficially similar brachiopods, the plane of symmetry passes between the valves, rather than through the valves. The head is reduced to a mouth and the foot is well-developed, typically functioning in burrowing. Unlike most of molluscan groups, the radula is absent. As in other molluscs, the shell is lined by the mantle and there is a cavity at one end, the mantle cavity, which houses the gills. The majority of bivalves are suspension feeders and the gills are modified into feeding structures (they also retain their respiratory function). The gills possess tracts of cilia. Some cilia set up currents in and out of the shell (through tubular structures termed siphons), whereas other tracts of cilia are responsible for trapping food (typically with the aid of mucus) and transporting it towards the mouth via flap-like structures termed palps (in the diagram below, the solid black arrows show the path of the water over the gills and the dashed arrows show the direction of food transport). -

What Do Byssus and Crimson Imply About the Hebrew Bible and the Septuagint? 1
BibAn 8/3 (2018) 297-317 297 What Do Byssus and Crimson Imply about the Hebrew Bible and the Septuagint? 1 ANNA RAMBIERT-KWAŚNIEWSKA Instytut Nauk Biblijnych, Papieski Wydział Teologiczny we Wrocławiu e-mail: [email protected] ORCID: 0000-0002-9491-6786 SUMMARY: Fabrics such as byssus and crimson wool can provide us with a surprisingly large amount of information about the circumstances of Biblical books origin. Analysis of lexis related to mentioned textiles, present in Exodus and in Chronicles, allows to notice a meaningful change, occurring in technical biblical terminology. In Exodus, whilst in Chronicles naming ,ּתֹולַ תעַ ׁשָ נִי and crimson – by ׁשֵ ׁש byssus is represented by ,Analysis of etymology leads to the conclusion . ַּכ רְ מִ י ל and ּבּוץ is changed respectively to that textiles mentioned in Torah belong to an early vocabulary, created in the times before the exile, in Chronicles, on the other hand, encountered terms belong to peri- od of Babylonian captivity or after the exile. Confrontation of these textiles with the other, non-biblical sources, provides a confirmation of proposed dating and facilitates identification of mysterious biblical byssus, which turns out to be a very thin linen and not – as sometimes confused – sea silk. Juxtaposition of the Greek equivalents allows to draw a conclusion, that terminology of both Torah and Chronicles was known to the and ׁשֵ ׁש :LXX translators, who – despite a clear caesura – translate terms consequently .to κόκκινος ַּכ רְ מִ י ל and ּתֹולַ תעַ ׁשָ נִי to βύσσος/βύσσινος, and ּבּוץ K EYWORDS: byssus, crimson, textiles, colors, fabric, Exodus, Chronicles, Septuagint, Hebrew Bible he research on the Biblical material culture constitutes mainly the archeological Tdomain.