Views & Reviews Demyelinating Disease After Neurologically
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Vaccination and Demyelination: Is There a Link? Examples with Anti
Vaccination and demyelination : Is there a link? Examples with anti-hepatitis B and papillomavirus vaccines Julie Mouchet Le Moal To cite this version: Julie Mouchet Le Moal. Vaccination and demyelination : Is there a link? Examples with anti-hepatitis B and papillomavirus vaccines. Human health and pathology. Université de Bordeaux, 2019. English. NNT : 2019BORD0015. tel-02134582 HAL Id: tel-02134582 https://tel.archives-ouvertes.fr/tel-02134582 Submitted on 20 May 2019 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. THÈSE PRÉSENTÉE POUR OBTENIR LE GRADE DE DOCTEUR DE L’UNIVERSITÉ DE BORDEAUX ÉCOLE DOCTORALE : Sociétés, Politique, Santé Publique (SP2) SPÉCIALITÉ : Pharmacologie option Pharmaco-épidémiologie, Pharmacovigilance Par Julie MOUCHET LE MOAL VACCINATION ET RISQUE DE DEMYELINISATION : EXISTE-T-IL UN LIEN ? EXEMPLES DES VACCINS ANTI-HEPATITE B ET ANTI-PAPILLOMAVIRUS Sous la direction de : Monsieur le Professeur Bernard Bégaud Soutenue publiquement le 29 Janvier 2019 Composition du jury Président : Christophe TZOURIO, Professeur des Universités -

Clinical and MRI Clues and Pitfalls in the Diagnosis and Differential Diagnosis of Multiple Sclerosis
Clinical and MRI clues and pitfalls in the diagnosis and differential diagnosis of Multiple Sclerosis Aksel Siva, M.D. MS Clinic & Department Of Neurology Istanbul University Cerrahpaşa School of Medicine [email protected] MSParis2017 - 7th Joint ECTRIMS - ACTRIMS Meeting, 25 - 28 October 2017 Disclosure • Received research grants to my department from The Scientific and Technological Research Council Of Turkey - Health Sciences Research Grants numbers : 109S070 and 112S052.; and also unrestricted research grants from Merck-Serono and Novartis to our Clinical Neuroimmunology Unit • Honoraria or consultation fees and/or travel and registration coverage for attending several national or international congresses or symposia, from Merck Serono, Biogen Idec/Gen Pharma of Turkey, Novartis, Genzyme, Roche and Teva. • Educational presentations at programmes & symposia prepared by Excemed internationally and at national meetings and symposia sponsored by Bayer- Schering AG; Merck-Serono;. Novartis, Genzyme and Teva-Turkey; Biogen Idec/Gen Pharma of Turkey Introduction… • The incidence and prevalence rates of MS are increasing, so are the number of misdiagnosed cases as MS! • One major source of misdiagnosis is misinterpretation of nonspecific clinical and imaging findings and misapplication of MRI diagnostic criteria resulting in an overdiagnosis of MS! • The differential diagnosis of MS includes the MS spectrum and related disorders that covers subclinical & clinical MS phenotypes, MS variants and inflammatory astrocytopathies, as well as other Ab-associated atypical inflammatory-demyelinating syndromes • There are a number of systemic diseases in which either the clinical or MRI findings or both may mimic MS, which further cause confusion! Related publication *Siva A. Common Clinical and Imaging Conditions Misdiagnosed as Multiple Sclerosis. -

MR Images, Brain Lesions, and Deep Learning
applied sciences Review MR Images, Brain Lesions, and Deep Learning Darwin Castillo 1,2,3,* , Vasudevan Lakshminarayanan 2,4 and María José Rodríguez-Álvarez 3 1 Departamento de Química y Ciencias Exactas, Sección Fisicoquímica y Matemáticas, Universidad Técnica Particular de Loja, San Cayetano Alto s/n, Loja 11-01-608, Ecuador 2 Theoretical and Experimental Epistemology Lab, School of Optometry and Vision Science, University of Waterloo, Waterloo, ON N2L3G1, Canada; [email protected] 3 Instituto de Instrumentación para Imagen Molecular (i3M) Universitat Politècnica de València—Consejo Superior de Investigaciones Científicas (CSIC), E-46022 Valencia, Spain; [email protected] 4 Departments of Physics, Electrical and Computer Engineering and Systems Design Engineering, University of Waterloo, Waterloo, ON N2L3G1, Canada * Correspondence: [email protected]; Tel.: +593-07-370-1444 (ext. 3204) Featured Application: This review provides a critical review of deep/machine learning algo- rithms used in the identification of ischemic stroke and demyelinating brain diseases. It eval- uates their strengths and weaknesses when applied to real world clinical data. Abstract: Medical brain image analysis is a necessary step in computer-assisted/computer-aided diagnosis (CAD) systems. Advancements in both hardware and software in the past few years have led to improved segmentation and classification of various diseases. In the present work, we review the published literature on systems and algorithms that allow for classification, identification, and detection of white matter hyperintensities (WMHs) of brain magnetic resonance (MR) images, specifically in cases of ischemic stroke and demyelinating diseases. For the selection criteria, we used bibliometric networks. Of a total of 140 documents, we selected 38 articles that deal with the main objectives of this study. -

Demyelination: What Is It and Why Does It Happen?
Demyelination: What Is It and Why Does It Happen? • Causes • Symptoms • Types • MS and Demyelination • Treatment and Diagnosis • Vaccines • Takeaway What is demyelination? Nerves send and receive messages from every part of your body and process them in your brain. Nerves allow you to speak, see, feel, and think. Many nerves are coated in myelin. Myelin is an insulating material. When it’s worn away or damaged, nerves can deteriorate, causing problems in the brain and throughout the body. Damage to myelin around nerves is called demyelination. Nerves Nerves are made up of neurons. Neurons are composed of a cell body, dendrites, and an axon. The axon sends messages from one neuron to the next. The axon also connects neurons to other cells, such as muscle cells. Some axons are extremely short. Others are 3 feet long. Some axons are covered in myelin. Myelin protects the axons and helps carry axon messages as quickly as possible. Myelin Myelin is made of membrane layers that cover an axon. This is similar to the idea of an electrical wire with coating to protect the metal underneath. Myelin allows a nerve signal to travel faster. In unmyelinated neurons, a signal can travel along the nerves at about 1 meter per second. In a myelinated neuron, the signal can travel 100 meters per second. Certain diseases can damage myelin. Demyelination slows down messages sent along axons and causes the axon to deteriorate. Depending upon the location of the damage, axon loss can cause problems with feeling, moving, seeing, hearing, and thinking clearly. CAUSES Causes of demyelination Inflammation is the most common cause of myelin damage. -

Cerebrospinal Fluid Analysis in Multiple Sclerosis Diagnosis: an Update
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Archivio istituzionale della ricerca - Università di Palermo medicina Review Cerebrospinal Fluid Analysis in Multiple Sclerosis Diagnosis: An Update Bruna Lo Sasso 1, Luisa Agnello 1, Giulia Bivona 1 , Chiara Bellia 1 and Marcello Ciaccio 1,2,* 1 Institute of Clinical Biochemistry, Clinical Molecular Medicine and Laboratory Medicine, Department of Biomedicine, Neuroscience and Advanced Diagnostics, University of Palermo, 90100 Palermo, Italy; [email protected] (B.L.S.); [email protected] (L.A.); [email protected] (G.B.); [email protected] (C.B.) 2 Department Laboratory Medicine, University-Hospital, 90100 Palermo, Italy * Correspondence: [email protected]; Tel.: +39-091-23865701; Fax: +39-091-655-3275 Received: 25 March 2019; Accepted: 30 May 2019; Published: 4 June 2019 Abstract: Multiple sclerosis (MS) is an immune-mediated demyelinating disease of the central nervous system (CNS) with brain neurodegeneration. MS patients present heterogeneous clinical manifestations in which both genetic and environmental factors are involved. The diagnosis is very complex due to the high heterogeneity of the pathophysiology of the disease. The diagnostic criteria have been modified several times over the years. Basically, they include clinical symptoms, presence of typical lesions detected by magnetic resonance imaging (MRI), and laboratory findings. The analysis of cerebrospinal fluid (CSF) allows an evaluation of inflammatory processes circumscribed to the CNS and reflects changes in the immunological pattern due to the progression of the pathology, being fundamental in the diagnosis and monitoring of MS. The detection of the oligoclonal bands (OCBs) in both CSF and serum is recognized as the “gold standard” for laboratory diagnosis of MS, though presents analytical limitations. -
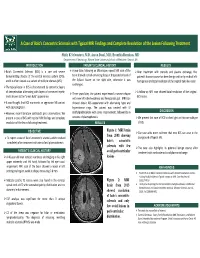
A Case of Balo's Concentric Sclerosis with Typical MRI Findings and Complete Resolution of the Lesion Followi
A Case of Balo’s Concentric Sclerosis with Typical MRI Findings and Complete Resolution of the Lesion Following Treatment Shitiz K Sriwastava, M.D, Aaron Desai, M.D, Evanthia Bernitsas, MD Department of Neurology, Wayne State University School of Medicine, Detroit, MI INTRODUCTION PATEINT’S CLINCIAL HISTORY RESULTS Balo’s Concentric Sclerosis (BCS) is a rare and severe A year later, following an MS relapse, repeat MRI scan of the After treatment with steroids and plasma exchange, the demyelinating disease of the central nervous system (CNS), brain showed a small enhancing focus at the posterior level of patient’s disease course has been benign with only residual left and it is often viewed as a variant of multiple sclerosis (MS). the Sylvian fissure on the right side, otherwise it was hemiparesis and total resolution of the original Balo‐like lesion. unchanged. The typical lesion in BCS is characterized by concentric layers of demyelination alternating with layers of preserved myelin A follow‐up MRI scan showed total resolution of the original Three years later, the patient experienced a severe relapse and is known as the “onion bulb” appearance. BCS lesion. with new left‐sided weakness and hemiparetic gait. MRI scan It was thought that BCS represents an aggressive MS variant showed classic BCS appearance with alternating hypo and with poor prognosis. hyperintense rings. The patient was treated with IV DISCUSSION However, recent literature contradicts prior observations. We methylprednisolone with some improvement, followed by 6 present a case of BCS with typical MRI findings and complete sessions of plasmapheresis. We present this case of BCS to shed light on this rare subtype resolution of the lesion following treatment. -

(Pre-Allison & Millar) Ipsen Criteria: 1939-48, Boston, MA
Early criteria (pre-Allison & Millar) Ipsen criteria: 1939-48, Boston, MA USA1 - Probable MS o Those cases with records presenting convincing evidence - Possible MS o Those cases whose evidence was more doubtful. Ipsen acknowledges these allocations are fairly arbitrary but also notes that absolutely certainty of diagnosis is impossible except by autopsy, a limitation we are similarly affected by even today. Westlund & Kurland: 1951, Winnipeg, MT Canada & New Orleans, LA USA2 Diagnostic groupings as follows, with no explicit requirements for each, rather deferring to the discretion of the examining neurologist: - Certain MS - Probable MS - Possible MS o In this class, the changes for and against MS were considered approximately even - Doubtful, unlikely or definitely-not MS2 Sutherland criteria: 1954, Northern Scotland3 - Probable MS o This category for patients in whom the history, the results of clinical examination and, where available, hospital investigations, indicated that the diagnosis of MS was beyond reasonable doubt. - Possible MS o Patients in whom a diagnosis of MS appears justifiable but in which the diagnosis could not be established beyond reasonable doubt; o This group also included patients who did not wish to be examined or could not be seen – review of hospital records for these patients suggested a diagnosis of MS - Rejected cases o Patients found to be suffering from a disease other than MS Allison & Millar Criteria and variants Allison & Millar criteria: 1954, Northern Ireland4 - Early disseminated sclerosis: 1) this category for patients with little in the way of symptomatic presentation but with a recent history consistent with disease onset, i.e. optic neuritis, ophthalmoplegia (double vision), vertigo, sensory problems like pins & needles or numbness, or motor problems like weakness. -

MS & Demyelinating Diseases.Pdf
JNS-14029; No. of Pages 29 ARTICLE IN PRESS Journal of the Neurological Sciences (2015) xxx–xxx Contents lists available at ScienceDirect Journal of the Neurological Sciences journal homepage: www.elsevier.com/locate/jns MS & Demyelinating Diseases 974 Tehran, Iran; bDepartment of Neurology, Iranian Center of Neurological WFN15-0282 Sciences, Tehran, Iran; cDepartment of Immunology, Tehran University MS & Demyelinating Diseases of Medical Sciences, Tehran, Iran Relationship between HLA-DRB1 genotype and clinical response to interferon-beta among Iranian multiple sclerosis patients Introduction: Multiple sclerosis is the most common autoimmune R. Abolfazlia, S. Samadzadeha, E. Tabibiana, A. Shakoorib, T. Sabokbarc, demyelinating disorder of central nervous system. The role of macro- S.H. Rahimi Dehgolana. aDepartment of Neurology, Tehran University phage in myelin destruction in this disabling disease is undeniable. of Medical Sciences, Tehran, Iran; bDepartment of Medical Genetics Chitotriosidase (Chit) is one of the mammalian enzymes synthesized and fi Cancer Institute, Tehran University of Medical Sciences, Tehran, Iran; secreted by speci cally activated macrophages. Recent studies indicate cNeurology &Neurosciences Research Center, Qom University of Medical that Chit may have a role in the pathogenesis of MS disease. Sciences, Qom, Iran Methods: We analyzed serum obtained from 40 relapsing remitting multiple sclerosis patients and 23 healthy individuals in Iranian Objective: To evaluate the relationship between HLA-DRB1 geno- population. Case and control group were sex and age matched, mean type, which has been proved to be more common in Iranian MS age were 31.92 and 33.54, respectively. In case group, sampling was patients, and clinical response to interferon-beta , which is the most done during attack before receiving steroid pulse or immunomodula- common immunotherapy for RRMS. -

Balo`'S Concentric Sclerosis: Still to Be Considered As a Variant of Multiple Sclerosis?
Neurol Sci (2015) 36:2277–2280 DOI 10.1007/s10072-015-2297-8 BRIEF COMMUNICATION Balo`’s concentric sclerosis: still to be considered as a variant of multiple sclerosis? Anna M. Pietroboni1 • Andrea Arighi1 • Milena A. De Riz1 • Laura Ghezzi1 • Alberto Calvi1 • Sabrina Avignone2 • Elisa Scola2 • Daniela Galimberti1 • Fabio Triulzi2 • Elio Scarpini1 Received: 26 March 2015 / Accepted: 16 June 2015 / Published online: 25 June 2015 Ó Springer-Verlag Italia 2015 Abstract Balo`’s concentric sclerosis (BCS) is considered a presentation of BCS. Moreover, these case reports suggest that rare demyelinating disease and regarded as an aggressive BCS may neither be rapidly progressive nor fatal and may be variant of multiple sclerosis (MS). We describe three cases considered part of the MS spectrum. In line with this hypoth- (one male and two females) with neuroimaging features sug- esis, current treatments for MS were effective in our patients. gestive of BCS and heterogeneous symptoms, with benign long-term clinical course upon treatment with natalizumab and Keywords Balo`’s concentric sclerosis (BCS) Á Multiple fingolimod. Neurological examination, blood and cere- sclerosis (MS) Á Clinically isolated syndrome (CIS) brospinal fluid analyses, brain and spinal cord magnetic reso- nance imaging (MRI) and brain proton magnetic resonance spectroscopy were performed. At onset, patient #1 showed Introduction predominant cognitive impairment with consciousness distur- bances; patient #2 presented with left hemiparesis; patient #3 Balo`’s concentric sclerosis (BCS) is a rare demyelinating demonstrated hesitance in speech and in written word pro- disease and it is regarded as one of the atypical forms of duction, along with right central facial palsy. -

ADEM) After Autologous Peripheral Blood Stem Cell Transplant for Non-Hodgkin’S Lymphoma
Bone Marrow Transplantation, (1999) 24, 1351–1354 1999 Stockton Press All rights reserved 0268–3369/99 $15.00 http://www.stockton-press.co.uk/bmt Case report Acute disseminated encephalomyelitis (ADEM) after autologous peripheral blood stem cell transplant for non-Hodgkin’s lymphoma A Re and R Giachetti Department of Hematology, University of Parma, Italy Summary: High-dose chemotherapy followed by autologous periph- eral blood stem cell transplantation (PBSCT) is a thera- Acute disseminated encephalomyelitis (ADEM) is a peutic intervention performed with increasing frequency for demyelinating disorder of the central nervous system hematologic and solid malignancies.4 The widespread use with an acute clinical onset and a wide variability in of this procedure depends on its safety and easy feasibility. severity and outcome. It usually follows a viral infection Mild organ toxicities and low incidence of life-threatening or an immunization and is thought to be immuno- complications are usually reported. Neurologic events are mediated. We report a case of ADEM with a dramatic frequently mild and reversible and usually secondary to clinical onset in an autologous peripheral blood stem injury to other organ systems.5 cell transplant (PBSCT) recipient for non-Hodgkin’s We report a case of ADEM in an autologous PBSCT lymphoma who developed the neurologic syndrome 12 recipient with non-Hodgkin’s lymphoma (NHL) who days after PBSC reinfusion. This is the first report of developed the syndrome on day ϩ12 after PBSC ADEM in the setting of autologous PBSCT, a thera- reinfusion, without any recognizable etiology. To our peutic procedure performed with increasing frequency knowledge, this is the first report of ADEM developing in a wide variety of hematologic and solid malignancies. -
Targeted Complement Inhibition at Synapses Prevents Microglial Synaptic Engulfment and Synapse Loss in Demyelinating Disease
Article Targeted Complement Inhibition at Synapses Prevents Microglial Synaptic Engulfment and Synapse Loss in Demyelinating Disease Graphical Abstract Authors Sebastian Werneburg, Jonathan Jung, Rejani B. Kunjamma, ..., Brian Popko, Daniel S. Reich, Dorothy P. Schafer Correspondence [email protected] In Brief The mechanisms underlying synaptic changes in multiple sclerosis (MS) remain unclear. Werneburg et al. identify microglia-mediated synapse engulfment and synapse loss in MS patients and multiple MS-relevant animal models. Synapse loss can occur early and prior to other MS-relevant pathology and is associated with synapse-localized complement C3. An AAV approach to inhibit C3 protects synapses and preserves circuit function. Highlights d Microglia engulf and eliminate synapses in the visual thalamus of MS patients d MS-relevant animal models show synapse engulfment and loss occur early in disease d Complement C3, but not C1q, localizes to synapses in demyelinating disease d AAV-Crry inhibits C3 and microglia-mediated synapse loss and preserves function Werneburg et al., 2020, Immunity 52, 167–182 January 14, 2020 ª 2019 Elsevier Inc. https://doi.org/10.1016/j.immuni.2019.12.004 Immunity Article Targeted Complement Inhibition at Synapses Prevents Microglial Synaptic Engulfment and Synapse Loss in Demyelinating Disease Sebastian Werneburg,1 Jonathan Jung,1 Rejani B. Kunjamma,2 Seung-Kwon Ha,3 Nicholas J. Luciano,3 Cory M. Willis,4 Guangping Gao,5,6,7 Natalia P. Biscola,8 Leif.A. Havton,8,9 Stephen J. Crocker,4 Brian Popko,2 -

Acute Transverse Myelitis and Acute Disseminated Encephalomyelitis
Acute Transverse Myelitis and Acute Disseminated Encephalomyelitis Ilana Kahn, MD* *Children’s National Health System, George Washington University Medical School, Washington, DC Education Gap Most pediatricians report lack of knowledge related to the understanding of the diagnosis and treatment of both acute disseminating encephalomyelitis and acute transvers myelitis. Pediatric providers should understand presenting symptoms, initial diagnostic testing, and acute treatment. Clinicians should know when to refer to a neurologist for evaluation of long-term treatment. Objectives After completing this article, readers should be able to: 1. Define and characterize acquired demyelinating syndromes. 2. Identify the prevalence, etiology, and clinical presentations of acute disseminating encephalomyelitis (ADEM) and acute transverse myelitis (ATM). 3. Initiate a diagnostic evaluation, including an evaluation for medical emergencies. 4. Make treatment decisions for acute management. 5. Counsel patients on long-term outcomes after ADEM and ATM. AUTHOR DISCLOSURE Dr Kahn has disclosed 6. Counsel patients on recurrence risks for multiphasic or chronic no financial relationships relevant to this article. This commentary does not contain a demyelinating diseases. discussion of an unapproved/investigative use of a commercial product/device. ABBREVIATIONS INTRODUCTION ADEM acute disseminated encephalomyelitis ADS acquired demyelinating syndrome Acquired demyelinating syndromes (ADSs) encompass a group of immune- AQP4 aquaporin-4 mediated disorders in which there is breakdown of the myelin sheath, the lipid- ATM acute transverse myelitis CNS central nervous system rich covering around the axon that increases conduction speed and metabolic CSF cerebrospinal fluid efficiency of the neuron. ADSs are characterized by a sudden onset of new IgG immunoglobulin G neurologic symptoms in concurrence with neuroimaging evidence of demyelin- IVIg intravenous immunoglobulin MOG myelin oligodendrocyte glycoprotein ation.