Inheritance of the Reduced Mitochondria of Giardia Intestinalis Is Coupled to the Flagellar Maturation Cycle
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Oxymonad Genome Displays Canonical Eukaryotic Complexity in the Absence of a Mitochondrion Anna Karnkowska,*,1,2 Sebastian C
The Oxymonad Genome Displays Canonical Eukaryotic Complexity in the Absence of a Mitochondrion Anna Karnkowska,*,1,2 Sebastian C. Treitli,1 Ondrej Brzon, 1 Lukas Novak,1 Vojtech Vacek,1 Petr Soukal,1 Lael D. Barlow,3 Emily K. Herman,3 Shweta V. Pipaliya,3 TomasPanek,4 David Zihala, 4 Romana Petrzelkova,4 Anzhelika Butenko,4 Laura Eme,5,6 Courtney W. Stairs,5,6 Andrew J. Roger,5 Marek Elias,4,7 Joel B. Dacks,3 and Vladimır Hampl*,1 1Department of Parasitology, BIOCEV, Faculty of Science, Charles University, Vestec, Czech Republic 2Department of Molecular Phylogenetics and Evolution, Faculty of Biology, Biological and Chemical Research Centre, University of Warsaw, Warsaw, Poland 3Division of Infectious Disease, Department of Medicine, University of Alberta, Edmonton, Canada 4Department of Biology and Ecology, Faculty of Science, University of Ostrava, Ostrava, Czech Republic Downloaded from https://academic.oup.com/mbe/article-abstract/36/10/2292/5525708 by guest on 13 January 2020 5Department of Biochemistry and Molecular Biology, Dalhousie University, Halifax, Canada 6Department of Cell and Molecular Biology, Uppsala University, Uppsala, Sweden 7Institute of Environmental Technologies, Faculty of Science, University of Ostrava, Ostrava, Czech Republic *Corresponding authors: E-mails: [email protected]; [email protected]. Associate editor: Fabia Ursula Battistuzzi Abstract The discovery that the protist Monocercomonoides exilis completely lacks mitochondria demonstrates that these organ- elles are not absolutely essential to eukaryotic cells. However, the degree to which the metabolism and cellular systems of this organism have adapted to the loss of mitochondria is unknown. Here, we report an extensive analysis of the M. -

Base J Originally Found in Kinetoplastida Is Also a Minor Constituent of Nuclear DNA of Euglena Gracilis
© 2000 Oxford University Press Nucleic Acids Research, 2000, Vol. 28, No. 16 3017–3021 Base J originally found in Kinetoplastida is also a minor constituent of nuclear DNA of Euglena gracilis Dennis Dooijes, Inês Chaves, Rudo Kieft, Anita Dirks-Mulder, William Martin1 and Piet Borst* Division of Molecular Biology and Centre for Biomedical Genetics, The Netherlands Cancer Institute, Plesmanlaan 121, 1066 CX Amsterdam, The Netherlands and 1Institute of Genetics, Technical University of Braunschweig, Spielmannstrasse 7, 38023 Braunschweig, Germany Received June 8, 2000; Accepted July 4, 2000 ABSTRACT DNA blots or by immunoprecipitation. The immunoprecipi- tated DNA can be analyzed by combined 32P-postlabeling and We have analyzed DNA of Euglena gracilis for the two-dimensional thin-layer chromatography (2D-TLC) experi- presence of the unusual minor base β-D-glucosyl- ments (6,7) to verify that J is present. Using these methods we hydroxymethyluracil or J, thus far only found in have shown that J is a conserved DNA modification in kineto- kinetoplastid flagellates and in Diplonema.Using plastid protozoans and is abundant in their telomeres (5). J was antibodies specific for J and post-labeling of DNA not detected in the animals, plants, or fungi tested, nor in a digests followed by two-dimensional thin-layer range of other simple eukaryotes, such as Plasmodium, chromatography of labeled nucleotides, we show Toxoplasma, Entamoeba, Trichomonas and Giardia (5). that ~0.2 mole percent of Euglena DNA consists of J, Outside the Kinetoplastida, J was only found in Diplonema,a an amount similar to that found in DNA of Trypano- small phagotrophic marine flagellate, in which we also soma brucei. -

Download the Abstract Book
1 Exploring the male-induced female reproduction of Schistosoma mansoni in a novel medium Jipeng Wang1, Rui Chen1, James Collins1 1) UT Southwestern Medical Center. Schistosomiasis is a neglected tropical disease caused by schistosome parasites that infect over 200 million people. The prodigious egg output of these parasites is the sole driver of pathology due to infection. Female schistosomes rely on continuous pairing with male worms to fuel the maturation of their reproductive organs, yet our understanding of their sexual reproduction is limited because egg production is not sustained for more than a few days in vitro. Here, we explore the process of male-stimulated female maturation in our newly developed ABC169 medium and demonstrate that physical contact with a male worm, and not insemination, is sufficient to induce female development and the production of viable parthenogenetic haploid embryos. By performing an RNAi screen for genes whose expression was enriched in the female reproductive organs, we identify a single nuclear hormone receptor that is required for differentiation and maturation of germ line stem cells in female gonad. Furthermore, we screen genes in non-reproductive tissues that maybe involved in mediating cell signaling during the male-female interplay and identify a transcription factor gli1 whose knockdown prevents male worms from inducing the female sexual maturation while having no effect on male:female pairing. Using RNA-seq, we characterize the gene expression changes of male worms after gli1 knockdown as well as the female transcriptomic changes after pairing with gli1-knockdown males. We are currently exploring the downstream genes of this transcription factor that may mediate the male stimulus associated with pairing. -

Unique Characteristics of the Kinetoplast DNA Replication
CHAPTER 2 Unique Characteristics of the Kinetoplast DNA Replication Machinery Provide Potential Drug Targets in Trypanosomatids Dotan Sela, Neta Milman, Irit Kapeller, Aviad Zick, Rachel Bezalel, Nurit Yaffe and Joseph Shlomai* Reevaluating the Kinetoplast as a Potential Target for Anti-Trypanosomal Drugs inetoplast DNA (kDNA) is a remarkable DNA structure found in the single mitohondrion of flagellated protozoa of the order Kinetoplastida. In various parasitic Kspecies of the family Trypanosomatidae, it consists of 5,000-10,000 duplex DNA minicircles (0.5-10 kb) and 25-50 maxicircles (20-40 kb), which are linked topologically into a two dimensional DNA network. Maxicircles encode for typical mitochondrial proteins and ribosomal RNA, whereas minicircles encode for guide RNA (gRNA) molecules that function in the editing of maxicircles’ mRNA transcripts. The replication of kDNA includes the dupli- cation of free detached minicircles and catenated maxicircles, and the generation of two prog- eny kDNA networks. It is catalyzed by an enzymatic machinery, consisting of kDNA replica- tion proteins that are located at defined sites flanking the kDNA disk in the mitochondrial matrix (for recent reviews on kDNA see refs. 1-8). The unusual structural features of kDNA and its mode of replication, make this system an attractive target for anti-trypanosomal and anti-leishmanial drugs. However, in evaluating the potential promise held in the development of drugs against mitochondrial targets in trypanosomatids, one has to consider the observations that dyskinetoplastic (Dk) bloodstream forms of trypanosomes survive and retain their infectivity, despite the substantial loss of their mitochondrial genome (recently reviewed in ref. 9). Survival of Dk strains has led to the notion that kDNA and mitochondrial functions are dispensable for certain stages of the life cycle of trypanosomatids. -
Prokaryotic and Eukaryotic Organisms Pdf
Prokaryotic and eukaryotic organisms pdf Continue There are two types of cells: prokaryotic and eukaryotic. In this section, we will examine the similarities and differences between the two types. The objectives of the training to identify features common to all cells contrast the composition and size of prokaryotic and eukaryotic cells fall into one of two broad categories: prokaryotic and eukaryotic. Single-celled organisms of the domains Of Bacteria and Archaea are classified as prokaryotes (pro - before; carion - core). Animal cells, plant cells, fungi and proteanists are eukaryotes (eu - truth). Components of prokaryotic cells All cells have four common components: (1) plasma membrane, external coating separating the inner part of the cell from the environment; (2) cytoplasm, consisting of a jelly-like area inside the cell in which other cellular components are located; (3) DNA, the genetic material of the cell; and (4) ribosomes, particles that synthesize proteins. However, prokaryotes differ from eukaryotic cells in several ways. Figure 1. This image shows the generalized structure of the prokaryotic cell. A prokaryotic cell is a simple single-celled organism that lacks a nucleus or any other membrane organella. Soon we will see that this is significantly different in eukaryotes. Prokaryotic DNA is found in the central part of the cell: a darkened area called a nucleoid (Figure 1). Unlike archaea and eukaryote, bacteria have a cell wall of peptidoglycan consisting of sugars and amino acids, and many of them have a polysaccharide capsule (Figure 1). The cell wall acts as an additional layer of protection, helps the cell maintain its shape and prevents dehydration. -
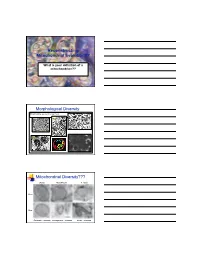
Reconstructing Mitochondrial Evolution?? Morphological Diversity Mitochondrial Diversity???
Reconstructing Mitochondrial Evolution?? What is your definition of a mitochondrion?? Morphological Diversity Mitochondria as we all know them: Suprarenal gland Liver cell Plasma cell Adrenal cortex Mitochondrial Diversity??? Chicken Neocallimastix T. foetus 100 nm 50 nm Entamoeba - mitosome microsporidian - mitosome Giardia - mitosome Reconstructing Evolution Mitochondrial evolution well established endosymbiotic theory α-proteobacterium - Rickettsia prowazekii Hydrogenosomal evolution No DNA NOW 2 examples Nyctotherus and Blastocystis (MLO) Several proteins similar to mitochondria Mitosome evolution No DNA Few proteins identified similar to mitochondria Origins via Endosymbiosis Current dogma - mitochondria and related Aerobic α-proteobacterium prokaryote gave rise to present day mitochondria. organelles arose just once in evolution Are hydrogenosomes and mitosomes of anaerobic protists derived from the same proto-mitochondrion? Evidence for: accumulating evidence for several proteins that are currently found in mitochondria - Proteins of Fe-S cluster formation. Scenario A Common ancestor facultative Scenario B organism? degenerate mitochondrion invoke lateral gene transfer from anaerobic prokaryotes Hypotheses for Mito Acquisition Mitochondrion-related Organelles 2 way to interpret this summary - can you think of both? Groups with mitochondrial homologues Hjort et al Phil. Trans. R. Soc. B 2010 365, 713-727 Origin of Hydrogenosomes (Mitosomes?) 1. a)Conversion of Mitochondria HYD b)Common ancestor with mitochondria ? HYD 2. Independent Origin α-proteobacterium ? - anaerobic bacterium HYD Organelles - origins and biogenesis Approaches: (1) Conduct phylogenetic analyses of similar proteins Hsp70 Fd Hsp60 Isc subunits (2) Examine protein targeting to the organelle matrix protein targeting membrane protein targeting (3) Characterize membrane/translocation components These components could have evolved as the endosymbiont was converted to organelle. Reveals evolutionary history. -

The Life Cycle of Trypanosoma (Nannomonas) Congolense in the Tsetse Fly Lori Peacock1,2, Simon Cook2,3, Vanessa Ferris1,2, Mick Bailey2 and Wendy Gibson1*
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by PubMed Central Peacock et al. Parasites & Vectors 2012, 5:109 http://www.parasitesandvectors.com/content/5/1/109 RESEARCH Open Access The life cycle of Trypanosoma (Nannomonas) congolense in the tsetse fly Lori Peacock1,2, Simon Cook2,3, Vanessa Ferris1,2, Mick Bailey2 and Wendy Gibson1* Abstract Background: The tsetse-transmitted African trypanosomes cause diseases of importance to the health of both humans and livestock. The life cycles of these trypanosomes in the fly were described in the last century, but comparatively few details are available for Trypanosoma (Nannomonas) congolense, despite the fact that it is probably the most prevalent and widespread pathogenic species for livestock in tropical Africa. When the fly takes up bloodstream form trypanosomes, the initial establishment of midgut infection and invasion of the proventriculus is much the same in T. congolense and T. brucei. However, the developmental pathways subsequently diverge, with production of infective metacyclics in the proboscis for T. congolense and in the salivary glands for T. brucei. Whereas events during migration from the proventriculus are understood for T. brucei, knowledge of the corresponding developmental pathway in T. congolense is rudimentary. The recent publication of the genome sequence makes it timely to re-investigate the life cycle of T. congolense. Methods: Experimental tsetse flies were fed an initial bloodmeal containing T. congolense strain 1/148 and dissected 2 to 78 days later. Trypanosomes recovered from the midgut, proventriculus, proboscis and cibarium were fixed and stained for digital image analysis. -

The Cytological Events and Molecular Control of Life Cycle Development of Trypanosoma Brucei in the Mammalian Bloodstream
pathogens Review The Cytological Events and Molecular Control of Life Cycle Development of Trypanosoma brucei in the Mammalian Bloodstream Eleanor Silvester †, Kirsty R. McWilliam † and Keith R. Matthews * Institute for Immunology and Infection Research, Centre for Immunity, Infection and Evolution, School of Biological Sciences, King’s Buildings, University of Edinburgh, Charlotte Auerbach Road, Edinburgh EH9 3FL, UK; [email protected] (E.S.); [email protected] (K.R.McW.) * Correspondence: [email protected]; Tel.: +44-131-651-3639 † These authors contributed equally to this work. Received: 23 May 2017; Accepted: 22 June 2017; Published: 28 June 2017 Abstract: African trypanosomes cause devastating disease in sub-Saharan Africa in humans and livestock. The parasite lives extracellularly within the bloodstream of mammalian hosts and is transmitted by blood-feeding tsetse flies. In the blood, trypanosomes exhibit two developmental forms: the slender form and the stumpy form. The slender form proliferates in the bloodstream, establishes the parasite numbers and avoids host immunity through antigenic variation. The stumpy form, in contrast, is non-proliferative and is adapted for transmission. Here, we overview the features of slender and stumpy form parasites in terms of their cytological and molecular characteristics and discuss how these contribute to their distinct biological functions. Thereafter, we describe the technical developments that have enabled recent discoveries that uncover how the slender to stumpy transition is enacted in molecular terms. Finally, we highlight new understanding of how control of the balance between slender and stumpy form parasites interfaces with other components of the infection dynamic of trypanosomes in their mammalian hosts. -

Novel Lineages of Oxymonad Flagellates from the Termite Porotermes Adamsoni (Stolotermitidae): the Genera Oxynympha and Termitim
Protist, Vol. 170, 125683, December 2019 http://www.elsevier.de/protis Published online date 21 October 2019 ORIGINAL PAPER Novel Lineages of Oxymonad Flagellates from the Termite Porotermes adamsoni (Stolotermitidae): the Genera Oxynympha and Termitimonas a,1 b a c b,1 Renate Radek , Katja Meuser , Samet Altinay , Nathan Lo , and Andreas Brune a Evolutionary Biology, Institute for Biology/Zoology, Freie Universität Berlin, 14195 Berlin, Germany b Research Group Insect Gut Microbiology and Symbiosis, Max Planck Institute for Terrestrial Microbiology, 35043 Marburg, Germany c School of Life and Environmental Sciences, The University of Sydney, Sydney, NSW 2006, Australia Submitted January 21, 2019; Accepted October 9, 2019 Monitoring Editor: Alastair Simpson The symbiotic gut flagellates of lower termites form host-specific consortia composed of Parabasalia and Oxymonadida. The analysis of their coevolution with termites is hampered by a lack of informa- tion, particularly on the flagellates colonizing the basal host lineages. To date, there are no reports on the presence of oxymonads in termites of the family Stolotermitidae. We discovered three novel, deep-branching lineages of oxymonads in a member of this family, the damp-wood termite Porotermes adamsoni. One tiny species (6–10 m), Termitimonas travisi, morphologically resembles members of the genus Monocercomonoides, but its SSU rRNA genes are highly dissimilar to recently published sequences of Polymastigidae from cockroaches and vertebrates. A second small species (9–13 m), Oxynympha loricata, has a slight phylogenetic affinity to members of the Saccinobaculidae, which are found exclusively in wood-feeding cockroaches of the genus Cryptocercus, the closest relatives of termites, but shows a combination of morphological features that is unprecedented in any oxymonad family. -

Visceral Leishmaniasis (Kala-Azar): Caused by Leishmania Donovani
تابع 2 أ.د / فاطمة إبراهيم سنبل أستاذ الميكروبيولوجيا الصيدلية • (Plate 2) • Life cycle of Balantidium coli Mastigophora • General characters : • This subphylum includes those protozoa that move actively by means of a Flagellum (or several flagella) during all or part of their life. In addition, the body has a definite form (not irregular as in amoebae) maintained by a firm pellicle on its outer surface. Some flagellates are devoid of a mouth opening but some have an opening or Cytostome through which food is ingested. They reproduced by longitudinal binary fission (not transverse fission as in case of B. coli). • Some have undulating membrane which appear to consist of highly modified flagellum. Flagellates have vesicular type of nucleus . • The parasitic flagellated protozoa fall into two categories with respect to the type of disease produced in humans : • a) Intestinal and genital flagellates : these are found only in the intestinal or genital tracts and their transmission does not require a biological vector and so pass directly from man to man usually enclosed in a cyst. They include : • - Giardia intestinalis (lamblia) • - Enteromonas hominis • - Trichomonas hominis • - Trichomonas tenax • - Trichomonas vaginalis • - Dientamoeba fragilis (amoeba-like flagellate) • The parasites are nearly all harmless commensals but two species, Giardia intestinalis and Trichomonas vaginalis are associated with inflammatory lesions in heavy infections and for practical purposes may be regarded as pathogenic . • b) Blood and tissue flagellates (haemoflagellates) : these infect the vascular system and various tissues and their transmission requires a biological vector. usually an arthropod (insect) in which they undergo a fresh cycle of alteration and multiplication before they can again infect man. -

Protozoan Parasites
Welcome to “PARA-SITE: an interactive multimedia electronic resource dedicated to parasitology”, developed as an educational initiative of the ASP (Australian Society of Parasitology Inc.) and the ARC/NHMRC (Australian Research Council/National Health and Medical Research Council) Research Network for Parasitology. PARA-SITE was designed to provide basic information about parasites causing disease in animals and people. It covers information on: parasite morphology (fundamental to taxonomy); host range (species specificity); site of infection (tissue/organ tropism); parasite pathogenicity (disease potential); modes of transmission (spread of infections); differential diagnosis (detection of infections); and treatment and control (cure and prevention). This website uses the following devices to access information in an interactive multimedia format: PARA-SIGHT life-cycle diagrams and photographs illustrating: > developmental stages > host range > sites of infection > modes of transmission > clinical consequences PARA-CITE textual description presenting: > general overviews for each parasite assemblage > detailed summaries for specific parasite taxa > host-parasite checklists Developed by Professor Peter O’Donoghue, Artwork & design by Lynn Pryor School of Chemistry & Molecular Biosciences The School of Biological Sciences Published by: Faculty of Science, The University of Queensland, Brisbane 4072 Australia [July, 2010] ISBN 978-1-8649999-1-4 http://parasite.org.au/ 1 Foreword In developing this resource, we considered it essential that -

Plants As Sources of Anti-Protozoal Compounds
PLANTS AS SOURCES OF ANTI- PROTOZOAL COMPOUNDS Thesis presented by Angela Paine for the degree of Doctor of Philosophy in the Faculty of Medicine of the University of London Department of Pharmacognosy The School of Pharmacy University of London 1995 ProQuest Number: 10104878 All rights reserved INFORMATION TO ALL USERS The quality of this reproduction is dependent upon the quality of the copy submitted. In the unlikely event that the author did not send a complete manuscript and there are missing pages, these will be noted. Also, if material had to be removed, a note will indicate the deletion. uest. ProQuest 10104878 Published by ProQuest LLC(2016). Copyright of the Dissertation is held by the Author. All rights reserved. This work is protected against unauthorized copying under Title 17, United States Code. Microform Edition © ProQuest LLC. ProQuest LLC 789 East Eisenhower Parkway P.O. Box 1346 Ann Arbor, Ml 48106-1346 dedicated to my late father Abstract The majority of the world's population relies on traditional medicine, mainly plant-based, for the treatment of disease. This study focuses on plant remedies used to treat tropical diseases caused by protozoan parasites. The following protozoal diseases: African trypanosomiasis, leishmaniasis. South American trypanosomiasis and malaria, and the traditional use of plant remedies in their treatment, are reviewed in a world wide context. In the present work, vector and mammalian forms of Trypanosoma b. brucei, the vector forms of Leishmania donovani and Trypanosoma cruzi and the mammalian forms of Plasmodium falciparum were maintained in culture in vitro in order to evaluate the activity of a series of plant extracts, pure natural products and synthetic analogues against these protozoan parasites in vitro.