Influence of Epilobium Angustifolium and Serenoa Repens Extracts on Cytochrome 2D2 and 3A1 Expression Level in Rats
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Pine Island Ridge Management Plan
Pine Island Ridge Conservation Management Plan Broward County Parks and Recreation May 2020 Update of 1999 Management Plan Table of Contents A. General Information ..............................................................................................................3 B. Natural and Cultural Resources ...........................................................................................8 C. Use of the Property ..............................................................................................................13 D. Management Activities ........................................................................................................18 E. Works Cited ..........................................................................................................................29 List of Tables Table 1. Management Goals…………………………………………………………………21 Table 2. Estimated Costs……………………………………………………………….........27 List of Attachments Appendix A. Pine Island Ridge Lease 4005……………………………………………... A-1 Appendix B. Property Deeds………….............................................................................. B-1 Appendix C. Pine Island Ridge Improvements………………………………………….. C-1 Appendix D. Conservation Lands within 10 miles of Pine Island Ridge Park………….. D-1 Appendix E. 1948 Aerial Photograph……………………………………………………. E-1 Appendix F. Development Agreement………………………………………………….. F-1 Appendix G. Plant Species Observed at Pine Island Ridge……………………………… G-1 Appendix H. Wildlife Species Observed at Pine Island Ridge ……... …………………. H-1 Appendix -
![Beta-Sitosterol [BSS] and Betasitosterol Glucoside [BSSG] As an Adjuvant in the Treatment of Pulmonary Tuberculosis Patients.” TB Weekly (4 Mar 1996)](https://docslib.b-cdn.net/cover/0902/beta-sitosterol-bss-and-betasitosterol-glucoside-bssg-as-an-adjuvant-in-the-treatment-of-pulmonary-tuberculosis-patients-tb-weekly-4-mar-1996-1630902.webp)
Beta-Sitosterol [BSS] and Betasitosterol Glucoside [BSSG] As an Adjuvant in the Treatment of Pulmonary Tuberculosis Patients.” TB Weekly (4 Mar 1996)
Saw Palmetto (Serenoa repens) and One of Its Constituent Sterols -Sitosterol [83-46-5] Review of Toxicological Literature Prepared for Errol Zeiger, Ph.D. National Institute of Environmental Health Sciences P.O. Box 12233 Research Triangle Park, North Carolina 27709 Contract No. N01-ES-65402 Submitted by Raymond Tice, Ph.D. Integrated Laboratory Systems P.O. Box 13501 Research Triangle Park, North Carolina 27709 November 1997 EXECUTIVE SUMMARY The nomination of saw palmetto and -sitosterol for testing is based on the potential for human exposure and the limited amount of toxicity and carcinogenicity data. Saw palmetto (Serenoa repens), a member of the palm family Arecaceae, is native to the West Indies and the Atlantic Coast of North America, from South Carolina to Florida. The plant may grow to a height of 20 feet (6.10 m), with leaves up to 3 feet (0.914 m) across. The berries are fleshy, about 0.75 inch (1.9 cm) in diameter, and blue-black in color. Saw palmetto berries contain sterols and lipids, including relatively high concentrations of free and bound sitosterols. The following chemicals have been identified in the berries: anthranilic acid, capric acid, caproic acid, caprylic acid, - carotene, ferulic acid, mannitol, -sitosterol, -sitosterol-D-glucoside, linoleic acid, myristic acid, oleic acid, palmitic acid, 1-monolaurin and 1-monomyristin. A number of other common plants (e.g., basil, corn, soybean) also contain -sitosterol. Saw palmetto extract has become the sixth best-selling herbal dietary supplement in the United States. In Europe, several pharmaceutical companies sell saw palmetto-based over-the-counter (OTC) drugs for treating benign prostatic hyperplasia (BPH). -

Dr. Duke's Phytochemical and Ethnobotanical Databases List of Plants for Diverticulitis
Dr. Duke's Phytochemical and Ethnobotanical Databases List of Plants for Diverticulitis Plant Chemical Count Activity Count Picrasma quassioides 1 1 Diospyros undulata 1 1 Plectranthus rugosus 1 1 Rhizophora mucronata 1 1 Pterospermum acerifolium 1 1 Salvia apiana 1 1 Duboisia leichhardtii 1 1 Erythroxylum zambesiacum 1 1 Ilex verticillata 1 1 Aglaia leptantha 1 1 Cephalotaxus harringtonii 2 1 Dianthus sp. 1 1 Larix laricina 1 1 Lycopodium serratum 1 1 Plectranthus trichocarpus 1 1 Helleborus niger 1 1 Rhododendron anthopogon 1 1 Vancouveria hexandra 1 1 Alnus rubra 1 1 Hedyotis lawsoniae 1 1 Garcinia xanthochymus 1 1 Acanthus ilicifolius 1 1 Pterospermum lanceaefolium 1 1 Simaba obovata 1 1 Salvia beckeri 1 1 Polypodium aureum 1 1 Sorbus americana 1 1 Plant Chemical Count Activity Count Banisteriopsis caapi 2 1 Cephalotaxus spp 1 1 Betula alba 1 1 Dianthus superbus 1 1 Astragalus gummifer 1 1 Citrus unshiu 1 1 Prunus pensylvanica 1 1 Alnus rugosa 1 1 Euphorbia broteri 1 1 Gmelina arborea 1 1 Caladium bicolor 1 1 Hippomane mancinella 1 1 Casearia arborea 1 1 Pterospermum suberifolium 1 1 Aralia spinosa 1 1 Fagonia cretica 1 1 Indigofera tinctoria 1 1 Ornithogalum umbellatum 1 1 Tripterygium wilfordiim 1 1 Haplophyton cimicidum 1 1 Betula alleghaniensis 1 1 Glechoma hirsuta 1 1 Hygrophila auriculata 1 1 Lasianthus chinensis 1 1 Bupleurum salicifolium 1 1 Acacia lenticularis 1 1 Euphorbia hermentiana 1 1 2 Plant Chemical Count Activity Count Rhus alata 1 1 Pterospermum xylocarpum 1 1 Thymus piperella 1 1 Castanopsis concinna 1 1 Senecio -

Bonita Bay Presentation Center Mitigation Plan Permit No
BONITA BAY PRESENTATION CENTER MITIGATION PLAN PERMIT NO. 36-00289-S, APPLICATION NO. 180907-3 JANUARY 18, 2019 PREPARED BY: TURRELL, HALL & ASSOCIATES, INC. MARIELLE KITCHENER 3584 EXCHANGE AVENUE NAPLES, FL 34104 (239) 643-0166- OFFICE, (239) 253-1860- CELL [email protected] Bonita Presentation Center- App. 180907-3 Mitigation Plan (1/18/19) 1.0 INTRODUCTION The Bonita Bay Presentation Center Project is a 1.408-acre parcel, which was previously developed in 1995 as a sales center in Bonita Bay with associated parking (Application 950726-1). Under the previous permit not all of the 1.41 upland acres was cleared and filled. Some areas remained untouched as native habitat. Several years ago, the sales center was removed, and the pavement scraped up and disposed of to make room for new development. The new applicant is requesting to construct two (2) structures on this parcel with associated stormwater management for pre-treatment. The new development would for the most part lie within the old clearing footprint. A very minor amount of new additional clearing is needed to accommodate the water management system and building bulkhead installation, but all new clearing and filling lies landward of the 1993 DER Binding Jurisdictional Line. The site is located on a peninsular island in the far west side of Bonita Bay with other development and golf courses. It is specifically situated in Section 29, Township 47 South, Range 25 East, Lee County, Florida (site address 4701 Bonita Bay Blvd., Bonita Springs, FL 34134). 2 Bonita Presentation Center- App. 180907-3 Mitigation Plan (1/18/19) 2.0 EXISTING CONDITIONS Habitats onsite as referenced in the FLUCCS Map are found described below. -

Induced Prostatic Hyperplasia in Sprague?Dawley Rats
JPP.book Page 995 Monday, May 28, 2007 12:41 PM JPP 2007, 59: 995–999 © 2007 The Authors Received December 18, 2006 Accepted March 30, 2007 DOI 10.1211/jpp.59.7.0012 Effects of coconut oil on testosterone-induced prostatic ISSN 0022-3573 hyperplasia in Sprague–Dawley rats María de Lourdes Arruzazabala, Vivian Molina, Rosa Más, Daisy Carbajal, David Marrero, Víctor González, Eduardo Rodríguez Abstract Benign prostatic hyperplasia (BPH) is the benign uncontrolled growth of the prostate gland, leading to difficulty with urination. Saw palmetto lipid extracts (SPLE), used to treat BPH, have been shown to inhibit prostate 5a-reductase, and some major components, such as lauric, myristic and oleic acids also inhibit this enzyme. Coconut oil (CO) is also rich in fatty acids, mainly lauric and myristic acids. We inves- tigated whether CO prevents testosterone-induced prostate hyperplasia (PH) in Sprague-Dawley rats. Animals were distributed into seven groups (10 rats each). A negative control group were injected with soya oil; six groups were injected with testosterone (3 mg kg−1) to induce PH: a positive control group, and five groups treated orally with SPLE (400 mg kg−1), CO or sunflower oil (SO) (400 and 800 mg kg−1). Treatments were given for 14 days. Rats were weighed before treatment and weekly thereafter. Rats were then killed and the prostates were removed and weighed. CO (400 and 800 mg kg−1), SPLE (400 mg kg−1) and SO at 800 mg kg−1, but not at 400 mg kg−1, significantly reduced the increase in pros- tate weight (PW) and PW:body weight (BW) ratio induced by testosterone (% inhibition 61.5%, 82.0%, 43.8% and 28.2%, respectively). -

The Physical Conditions and Age Indicated by the Flora of Ti-Ie Alum Bluff Formation
THE PHYSICAL CONDITIONS AND AGE INDICATED BY THE FLORA OF TI-IE ALUM BLUFF FORMATION. By EDWARD WILBER BERRY. INTRODUCTION. of the formation, is a thin series of interbedded The present paper has for its purpose the greenish sands and marls overlying the Oak description of a small flora collected from the Grove sand. It was named from Shoal River, Alum Bluff formation, representing a horizon in western Florida, and is not represented by a hitherto unrepresented paleobotanically in lithologic 'unit at Alum Bluff. southeastern North America, and the discus The following section was taken at the point sion of the bearing of this flora on the physical where the fossil plants were collected, near the conditions of deposition and the probable age lower end of the bluff and in the immediate 2 of the dcposi ts. vicinity of the section measured by Dall. It is deemed worthy of reproduction because it GEOLOGY OF THE DEPOSITS. differs in certain particulars from Dall's sec The Alum Bluff formation was named from tion. Still other sections from different parts the bluff of that name on the east bank of the of the bluff are given by Sellards and Gunter.3 Apalachicola River, about 25 miles below Chat Section at Alum Bluff, Fla. tahoochee or River Junction, in Liberty County, Fla.t (See Pl. VII, B, p. 56.) It is, according Pleistocene (?): Feet. to present knowledge, the uppermost formation Light-colored ferruginous, rather loose sands. 9 Hard reddish clay ______ ... __ ... _.. __ . _. 2 of the Apalachicola group. It comprises three Variegated reddish and yellowish ferruginous members, which, named in ascending order, are sands ___ . -

Public Notice
DEPARTMENT OF THE ARMY JACKSONVILLE DISTRICT CORPS OF ENGINEERS COCOA PERMIT SECTION 400 HIGH POINT DRIVE COCOA, FLORIDA 32926 REPLY TO ATTENTION OF Regulatory Division November 14, 2017 North Permits Branch Cocoa Permits Section PUBLIC NOTICE Permit Application Number SAJ-2004-01749 (SP-JSC) TO WHOM IT MAY CONCERN: The Jacksonville District of the U.S. Army Corps of Engineers (Corps) has received an application for a Department of the Army permit pursuant to Section 404 of the Clean Water Act (33 U.S.C. §1344) as described below: APPLICANT: KB Home Jacksonville, LLC c/o Maston Crapps 10475 Fortune Parkway #100 Jacksonville, Florida 32256 WATERWAY AND LOCATION: The 553.3 ± acre Willow Creek Estates project would affect waters of the United States associated with the Halfway Lake Basin (HUC 0308010108), which is part of the Middle St. Johns River Watershed. The project site is located east of the intersection of SR 407 and I-95 in Brevard County (Sections 4, 9 and 10, Township 23 South, Range 35 East), Florida. Directions to the site are as follows: From SR 407 in Titusville, proceed south on Grissom Parkway approximately 1.5 miles. The project site is to the west. APPROXIMATE CENTRAL COORDINATES: Latitude 28.49724º Longitude -80.82333º PROJECT PURPOSE: Basic: Residential construction. Overall: The overall project purpose is the development of single- and multi-family residential in Brevard County, Florida. EXISTING CONDITIONS: The site is connected to the St. Johns River floodplain by a culvert under I-95. The Willow Creek currently supports seven land use types/vegetative communities within its boundaries. -
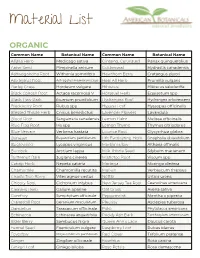
Material List
Material List ORGANIC Common Name Botanical Name Common Name Botanical Name Alfalfa Herb Medicago sativa Ginseng, Cultivated Panax quinquefolius Anise Seed Pimpinella anisum Goldenseal Hydrastis canadensis Ashwagandha Root Withania somnifera Hawthorn Berry Crataegus piperi Astragalus Root Astragalus membranaceus Heal All Herb Prunella vulgaris Barley Grass Hordeum vulgare Hibiscus Hibiscus sabdariffa Black Cohosh Root Actaea racemosa V Horsetail Herb Equisetum spp Black Haw Bark iburnum prunifolium Hydrangea Root Hydrangea arborescens Blackberry Root Rubus spp Hyssop Leaf Hyssopus officinalis Blessed Thistle Herb Cnicus benedictus Lavender Flowers Lavandula Blood Root Sanguinaria canadensis Lemon Balm Melissa officinalis Blue Flag Root Iris spp Lemon Thyme Thymus citriodorus Blue Vervain Verbena hastata Licorice Root Glycyrrhiza glabra Boneset Eupatorium perfoliatum Life Everlasting Herb Gnaphaliu obiusifolium Bugleweed Lycopus virginicus Marshmallow Althaea offinalis Burdock Arctium lappa Milk Thistle Seed Silybum marianum Butternut Bark Juglans cinerea Mistletoe Root Viscum spp Catnip Herb Nepeta cataria Moringa Moringa oleifera Chamomile Chamomilla recutita Mullein Verbascum thapsus Chaste Tree Berry Vitex agnus-castus Nettle Urtica urens Chicory Root Cichorium intybus New Jersey Tea Root Ceanothus americana Cleavers Herb Galium aparine Oat Grass Avena sativa Comfrey Symphytum officinale Peppermint Mentha x piperita Cranesbill Root Geranium maculatum Pleurisy Asclepias tuberosa Dandelion Taraxacum officinale Poke Phytolacca americana -

Serenoa Repens Saw Palmetto1 Edward F
FPS-547 Serenoa repens Saw Palmetto1 Edward F. Gilman2 Introduction Common name(s): saw palmetto Family: Aracaceae Saw palmetto is an extremely sturdy palm with great Plant type: palm textural interest that blends in well with natural or seaside USDA hardiness zones: 8 through 11 (Fig. 2) landscapes (Fig. 1). This low, clumping, bushy palm has Planting month for zone 8: year round large, fan-shaped leaves and multiple trunks that creep Planting month for zone 9: year round along the ground, creating a dense ground cover. Most saw Planting month for zone 10 and 11: year round palmettos have green leaves, but a form with blue leaves Origin: native to Florida can be found along the southeast coast of Florida. Three- Uses: mass planting; specimen; naturalizing; border; foot-long flower stalks appear in spring, covered with small, reclamation plant; accent; ground cover; attracts butterflies yellow-white, fragrant flowers, the source of a commercial Availability: somewhat available, may have to go out of the high-grade honey. The flowers are followed by small, yellow region to find the plant berries that turn black, ripening August through October. These berries are an important food source for many mammals and birds. Figure 2. Shaded area represents potential planting range. Figure 1. Saw palmetto Description General Information Growth rate: slow Scientific name: Serenoa repens Height: 5 to 10 feet Pronunciation: sair-ren-NOE-uh REE-penz Spread: 4 to 10 feet 1. This document is FPS-547, one of a series of the Environmental Horticulture Department, UF/IFAS Extension. Original publication date October 1999. -

Evaluation of Laurel Wilt Disease in Georgia: Progression in Redbay and Sassafras – 2008-2010
Evaluation of Laurel Wilt Disease in Georgia: Progression in Redbay and Sassafras – 2008-2010 A program of the Georgia Forestry Commission with support from the USDA Forest Service – Forest Health Protection Region 8 Evaluation of Laurel Wilt Disease in Georgia: Progression in Redbay and Sassafras – 2008-2010 By: R. Scott Cameron, Chip Bates, and James Johnson Georgia Forestry Commission December 2010 Cover photo: A unique high density stand of large redbay devastated by laurel wilt disease near Claxton, GA, photographed on 2/11/2009. ii Table of Contents Background ......................................................................................................................................................... 1 Objectives............................................................................................................................................................ 1 Methods ............................................................................................................................................................... 1 LWD Distribution and Spread in Georgia ................................................................................................ 1 Long-term Monitoring Plots ........................................................................................................................ 1 Plot distribution and classification ......................................................................................................... 2 Plot installation and data collection methods ...................................................................................... -

Implications of Embryo Desiccation Tolerance, Seed Dormancy
IMPLICATIONS OF EMBRYO DESICCATION TOLERANCE, SEED DORMANCY, AND SEED DAMAGE FOR CONSERVATION OF PRITCHARDIA PALMS ENDEMIC TO HA WAIT A DISSERTATION SUBMITTED TO THE GRADUATE DIVISION OF THE UNIVERSITY OF HA WAIT IN PARTIAL FULLFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF DOCTOR OF PHILOSOPHY IN TROPICAL PLANT AND SOIL SCIENCES (ECOLOGY, EVOLUTION, AND CONSERVATION BIOLOGY) MAY 2006 By: Hector E. Perez Dissertation Committee: Richard Criley, Chairperson Kent Kobayahsi Carol Baskin Mike Maunder Christina Walters Donald Drake DEDICATION This dissertation is dedicated to the memory of Amparo Claramunt. Her blessings allowed me to pursue my ambitions. Ill ACKNOWLEDGMENTS I would like to thank the National Science Foundation Grades K-12 Teaching Fellowship Program; the Ecology, Evolution, and Conservation interdisciplinary specialization; the ARCS Foundation; and the Haruyuki Kamemoto Scholarship for funding for my research and academic endeavors, I am thankful to all my committee members for their mentoring and support during my studies. I am grateful for the invaluable technical and logistical support provided by Lisa Hill, Jennifer Crane, and John Waddell of the National Center for Germplasm Preservation Research (USDA- ARS, Ft. Collins, CO). I thank Drs. Nancy Chen, Sung-Eun Lee, Qingxiao Li, Loren Gautz, Robert Pauli, Yoneo Sagawa, and David Webb, and Tina Carvalho for their technical assistance, use of lab space, and equipment. The Army Natural Resources, Environmental Division, Conservation and Restoration Branch also provided logistical support for preliminary studies. 1 express my gratitude to Mr. Alvin Yoshinaga for all his help and hospitality. 1 thank my family and friends for all the love and support they provided, whether local or long-distance. -

P2828 Cold Injury to Palms
Cold Injury to Palms Every few winters, arctic fronts plunge frigid tempera- Care of the Fronds tures into our state, harming our landscape plants. Some After a frost or cold injury, you may notice that the of the plants most afflicted by the ravages of winter are lower, older fronds are more damaged by cold than the palms, so let’s think about a little first aid for these plants younger, upper fronds. Regardless of how they appear, that add enjoyment to our landscapes. it is important to keep the fronds on as long as possible, at the least until there is no longer any green left in them Selecting the Palm and all chance of further cold is gone. Remember that the and Its Location fronds also shelter the palm heart from direct sun, so re- The amount of cold injury to a palm depends upon the taining old fronds until new fronds have grown in is ben- type (or species), how well it has adapted to its landscape, eficial. the duration of the cold temperatures, and the location of Palms grow slowly and commonly require 7 months plants or buildings around the palm that may offer some to begin to re-leaf after cold injury. When they do produce protection. When selecting a palm for your landscape, new fronds, the first several are likely to be deformed or choose a species that is well adapted to our climate, not to discolored. When removing dead fronds from the palm, central Florida’s (see Tables 1 and 2). Secondarily, try to after two or three new leaves have formed, remember not buy locally.