Microbial Enzyme Activity, Nutrient Uptake and Nutrient Limitation in Forested Streams
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Does Dietary Fiber Affect the Levels of Nutritional Components After Feed Formulation?
fibers Article Does Dietary Fiber Affect the Levels of Nutritional Components after Feed Formulation? Seidu Adams 1 ID , Cornelius Tlotliso Sello 2, Gui-Xin Qin 1,3,4, Dongsheng Che 1,3,4,* and Rui Han 1,3,4 1 College of Animal Science and Technology, Jilin Agricultural University, Changchun 130118, China; [email protected] (S.A.); [email protected] (G.-X.Q.); [email protected] (R.H.) 2 College of Animal Science and Technology, Department of Animal Genetics, Breeding and Reproduction, Jilin Agricultural University, Changchun 130118, China; [email protected] 3 Key Laboratory of Animal Production, Product Quality and Security, Jilin Agricultural University, Ministry of Education, Changchun 130118, China 4 Jilin Provincial Key Laboratory of Animal Nutrition and Feed Science, Jilin Agricultural University, Changchun 130118, China * Correspondence: [email protected]; Tel.: +86-136-4431-9554 Received: 12 January 2018; Accepted: 25 April 2018; Published: 7 May 2018 Abstract: Studies on dietary fiber and nutrient bioavailability have gained an increasing interest in both human and animal nutrition. Questions are increasingly being asked regarding the faith of nutrient components such as proteins, minerals, vitamins, and lipids after feed formulation. The aim of this review is to evaluate the evidence with the perspective of fiber usage in feed formulation. The consumption of dietary fiber may affect the absorption of nutrients in different ways. The physicochemical factors of dietary fiber, such as fermentation, bulking ability, binding ability, viscosity and gel formation, water-holding capacity and solubility affect nutrient absorption. The dietary fiber intake influences the different methods in which nutrients are absorbed. -

Macronutrients and Human Health for the 21St Century
nutrients Editorial Macronutrients and Human Health for the 21st Century Bernard J. Venn Department of Human Nutrition, University of Otago, Dunedin 9054, New Zealand; [email protected] Received: 30 July 2020; Accepted: 4 August 2020; Published: 7 August 2020 Abstract: Fat, protein and carbohydrate are essential macronutrients. Various organisations have made recommendations as to the energy contribution that each of these components makes to our overall diet. The extent of food refining and the ability of food systems to support future populations may also impact on how macronutrients contribute to our diet. In this Special Issue, we are calling for manuscripts from all disciplines to provide a broad-ranging discussion on macronutrients and health from personal, public and planetary perspectives. Keywords: macronutrient; fat; protein; carbohydrate; acceptable macronutrient distribution range; starch; sustainability The macronutrients, fat, protein and carbohydrate provide energy and essential components to sustain life. Fat is composed of glycerol and fatty acids; protein is an agglomeration of amino acids; and carbohydrate is simple sugars occurring either as monosaccharides or chains of connected monosaccharides (e.g., starch) whose bonds are either hydrolysed in the human small intestine to monosaccharides or are resistant to hydrolysis (dietary fibre). To maintain longevity and health, a combination of these macronutrients is required in our diet. It is elusive as to whether there is a combination of macronutrients that provides optimal health. When expressed as a percentage of energy to the diet, human populations have historically survived on diets with greatly differing proportions of these macronutrients. For example, the animal-based diet of an Alaskan Inuit group was found to comprise 33% protein, 41% fat and 26% carbohydrate [1]. -

The Science and Scope of Nutrition
The Science and Scope 1 of Nutrition LEARNING OBJECTIVES Define the scope and science of nutrition ( Infographic 1.1 ) Explain the connection between nutrition and chronic disease ( Infographic 1.2 ) Define and identify the major macronutrients and micronutrients ( Infographic 1.3 and Infographic 1.4 ) Summarize the purpose of the Dietary Reference Intake (DRI) values ( Infographic 1.5 ) Distinguish between the different types of DRI values and what each represents ( Infographic 1.6 and Infographic 1.7 ) Understand/explain the basis of the scientific method and how it is CHAPTER used in nutrition research ( Infographic 1.8 ) Describe three types of experimental design and the primary advantages of each ( Infographic 1.9 ) Describe reliable sources of nutrition information ( Infographic 1.10 ) t was the final months of World War II, and the Dutch people were starving. As a last-ditch effort to hold on to the Netherlands, the Germans had blocked the transport of food SPL/Science Source SPL/Science Ifrom the Netherlands eastern rural areas to its west- Exploring the ern cities. As a result, people were eating only a few hundred calories per day, typically a couple of small Science of slices of bread and potatoes. Some people used paper to thicken soup. This period, now known as the Dutch Hunger Winter, lasted from October 1944 Nutrition until the Netherlands was liberated in May 1945. 1 Copyright © W. H. Freeman and Company. Distributed by W. H. Freeman and Company strictly for use with its products. Not for redistribution. 02_POP_14867_ch01_001_023.indd 1 10/30/18 10:27 AM 1 THE SCIENCE AND SCOPE OF NUTRITION Thirty years later, a husband and wife it appeared as if a woman’s diet during preg- team of scientists at Columbia University nancy could have a strong influence on the decided to investigate whether these extreme weight of her child. -

Vitamin and Mineral Requirements in Human Nutrition
P000i-00xx 3/12/05 8:54 PM Page i Vitamin and mineral requirements in human nutrition Second edition VITPR 3/12/05 16:50 Page ii WHO Library Cataloguing-in-Publication Data Joint FAO/WHO Expert Consultation on Human Vitamin and Mineral Requirements (1998 : Bangkok, Thailand). Vitamin and mineral requirements in human nutrition : report of a joint FAO/WHO expert consultation, Bangkok, Thailand, 21–30 September 1998. 1.Vitamins — standards 2.Micronutrients — standards 3.Trace elements — standards 4.Deficiency diseases — diet therapy 5.Nutritional requirements I.Title. ISBN 92 4 154612 3 (LC/NLM Classification: QU 145) © World Health Organization and Food and Agriculture Organization of the United Nations 2004 All rights reserved. Publications of the World Health Organization can be obtained from Market- ing and Dissemination, World Health Organization, 20 Avenue Appia, 1211 Geneva 27, Switzerland (tel: +41 22 791 2476; fax: +41 22 791 4857; e-mail: [email protected]). Requests for permis- sion to reproduce or translate WHO publications — whether for sale or for noncommercial distri- bution — should be addressed to Publications, at the above address (fax: +41 22 791 4806; e-mail: [email protected]), or to Chief, Publishing and Multimedia Service, Information Division, Food and Agriculture Organization of the United Nations, 00100 Rome, Italy. The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the World Health Organization and the Food and Agriculture Organization of the United Nations concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. -

The Nutrition Contribution of Dietary Supplements on Total Nutrient Intake in Children and Adolescents
European Journal of Clinical Nutrition (2016) 70, 257–261 © 2016 Macmillan Publishers Limited All rights reserved 0954-3007/16 www.nature.com/ejcn ORIGINAL ARTICLE The nutrition contribution of dietary supplements on total nutrient intake in children and adolescents M Kang1, DW Kim2, H Lee3,YJLee3, HJ Jung1, H-Y Paik1,3 and YJ Song4 BACKGROUND/OBJECTIVES: The use of dietary supplements (DSs) by children and adolescents is increasing. The aim of this study was to identify the characteristics of DS users and examine the nutritional contributions of DSs to total nutrient intakes in children and adolescents, using data obtained from a national survey. SUBJECTS/METHODS: In total, 3134 subjects aged 9–18 years who participated in the 4th Korea National Health and Nutrition Examination Survey (2007–2009) were selected; the survey included 24-h recall questions on food intake and questions on DS use over the past year. Nutrient intakes from DSs were calculated using the aid of a label-based database on such supplements, and individual total nutrient intakes were derived by combining information on the foods and DSs consumed by each subject. RESULTS: There were 895 DS users (28.5%), 85.2% of whom (n = 577) had complete DS nutrient information and were therefore defined as identified-DS users. Identified-DS users were slightly younger and had a greater household income and better nutritional knowledge than did non-users. The most frequently consumed type of supplement was a ‘multivitamin and minerals’ complex. For total nutrient intake, identified-DS users had a significantly higher intake of most of the nutrients, except for macronutrient and sodium than non-users. -

Human Vitamin and Mineral Requirements
Human Vitamin and Mineral Requirements Report of a joint FAO/WHO expert consultation Bangkok, Thailand Food and Agriculture Organization of the United Nations World Health Organization Food and Nutrition Division FAO Rome The designations employed and the presentation of material in this information product do not imply the expression of any opinion whatsoever on the part of the Food and Agriculture Organization of the United Nations concerning the legal status of any country, territory, city or area or of its authorities, or concern- ing the delimitation of its frontiers or boundaries. All rights reserved. Reproduction and dissemination of material in this information product for educational or other non-commercial purposes are authorized without any prior written permission from the copyright holders provided the source is fully acknowledged. Reproduction of material in this information product for resale or other commercial purposes is prohibited without written permission of the copyright holders. Applications for such permission should be addressed to the Chief, Publishing and Multimedia Service, Information Division, FAO, Viale delle Terme di Caracalla, 00100 Rome, Italy or by e-mail to [email protected] © FAO 2001 FAO/WHO expert consultation on human vitamin and mineral requirements iii Foreword he report of this joint FAO/WHO expert consultation on human vitamin and mineral requirements has been long in coming. The consultation was held in Bangkok in TSeptember 1998, and much of the delay in the publication of the report has been due to controversy related to final agreement about the recommendations for some of the micronutrients. A priori one would not anticipate that an evidence based process and a topic such as this is likely to be controversial. -

Nutritional Value of Spirulina and Its Use in the Preparation of Some Complementary Baby Food Formulas
Available online at http://journal-of-agroalimentary.ro Journal of Agroalimentary Processes and Journal of Agroalimentary Processes and Technologies Technologies 2014, 20(4), 330-350 Nutritional value of spirulina and its use in the preparation of some complementary baby food formulas Ashraf M. Sharoba Food Sci. Dept., Fac. of Agric., Moshtohor, Benha Univ., Egypt Received: 26 September 2014; Accepted:03 Octomber 2014 .____________________________________________________________________________________ Abstract In this study use the spirulina which is one of the blue-green algae rich in protein 62.84% and contains a high proportion of essential amino acids (38.46% of the protein) and a source of naturally rich in vitamins especially vitamin B complex such as vitamin B12 (175 µg / 10 g) and folic acid (9.92 mg / 100 g), which helps the growth and nutrition of the child brain, also rich in calcium and iron it containing (922.28 and 273.2 mg / 100 g, respectively) to protect against osteoporosis and blood diseases as well as a high percentage of natural fibers. So, the spirulina is useful and necessary for the growth of infants and very suitable for children, especially in the growth phase, the elderly and the visually appetite. It also, helps a lot in cases of general weakness, anemia and chronic constipation. Spirulina contain an selenium element (0.0393 mg/100 g) and many of the phytopigments such as chlorophyll and phycocyanin (1.56% and 14.647%), and those seen as a powerful antioxidant. Finally, spirulina called the ideal food for mankind and the World Health Organization considered its "super food" and the best food for the future because of its nutritional value is very high. -

Focus on Nutrient Dense Foods and Beverages
COOPERATIVE EXTENSION SERVICE • UNIVERSITY OF KENTUCKY COLLEGE OF AGRICULTURE, LEXINGTON, KY, 40546 FCS3-559 Making Healthy Lifestyle Choices Focus on Nutrient-Dense Foods and Beverages Ingrid Adams, Department of Nutrition and Food Science ach day we are bombarded with a range of food choices—chips, vegetables, candy, and ice cream. We decide whether we should have doughnuts and coffee for breakfast or oatmeal with blueberries? ShouldE we have pizza or a tossed salad for lunch? Many of us eat whatever we Nutrient-dense foods have more want and make food decisions nutrients per calorie than foods with little thought about how that are not nutrient dense. foods benefit our bodies. The Nutrient-dense foods contain a Healthy Eating Index is a tool small portion of their calories developed by the Center for Nu- from fat, sugar, or refined grains trition Policy and Promotion to and have more vitamins, miner- assess the nutritional quality of als, and protein. the diet of Americans. Informa- tion gathered from this docu- Why is Nutrient-Dense When we make ment shows that approximately Food the Best Choice? 74 percent of Americans need to We all need a certain amount nutrient-dense improve their diets. of calories and nutrients each choices we get the Even though many of us do not day in order to be healthy. The always choose the healthiest amount of calories and nutri- nutrients we need foods, we generally have good ents needed are based on our intentions. Often we do not have age, sex, activity level, and how without consuming the time, money, or skills that healthy we are. -

Nutrient Value of Some Common Foods
Nutrient Value of Some Common Foods Nutrient-Value_e.indd 1 3/5/2008 12:36:28 AM Nutrient Value of Some Common Foods Nutrient Value of Some Common Foods Health Canada is the federal department responsible for helping Canadians maintain and improve their health. We assess the safety of drugs and many consumer products, help improve the safety of food, and provide information to Canadians to help them make healthy decisions. We provide health services to First Nations people and to Inuit communities. We work with the provinces to ensure our health care system serves the needs of Canadians. Published by authority of the Minister of Health. Nutrient Value of Some Common Foods is available on Internet at the following address: www.healthcanada.gc.ca/cnf Également disponible en français sous le titre : Valeur nutritive de quelques aliments usuels This publication can be made available by request on diskette, large print, audio-cassette and braille. For further information or to obtain additional copies, please contact: Publications Health Canada Ottawa, Ontario K1A 0K9 Tel.: (613) 954-5995 or 1-866-225-0709 Fax: (613) 941-5366 E-Mail: [email protected] © Her Majesty the Queen in Right of Canada, represented by the Minister of Health Canada, 2008 Require permission at all times HC Pub.: 4771 Cat.: H164-49/2008E-PDF ISBN: 978-0-662-48082-2 1 Nutrient-Value_e.indd 1 3/5/2008 12:36:54 AM Nutrient Value of Some Common Foods Introduction As Canadians recognize the crucial role of nutrition in the maintenance of good health, they increasingly seek information regarding the nutrient density of foods on the Canadian market. -

Spirulina Microalgae and Brain Health: a Scoping Review of Experimental and Clinical Evidence
marine drugs Review Spirulina Microalgae and Brain Health: A Scoping Review of Experimental and Clinical Evidence Vincenzo Sorrenti 1,2,* , Davide Augusto Castagna 3, Stefano Fortinguerra 4 , Alessandro Buriani 2 , Giovanni Scapagnini 5 and Donald Craig Willcox 6,7 1 Department of Pharmaceutical and Pharmacological Sciences, University of Padova, 35131 Padova, Italy 2 Maria Paola Belloni Center for Personalized Medicine, Data Medica Group (Synlab Limited), 35100 Padova, Italy; [email protected] 3 MedicRiab srls Via Novara, 6, 36071 Arzignano, Italy; [email protected] 4 IRCCS SDN, 80143 Napoli, Italy; [email protected] 5 Department of Medicine and Health Sciences “V. Tiberio”, University of Molise, 86100 Campobasso, Italy; [email protected] 6 Department of Human Welfare, Okinawa International University, Ginowan 901-2701, Japan; [email protected] 7 Department of Research, Kuakini Medical Center, Honolulu, HI 96817, USA * Correspondence: [email protected] Abstract: Spirulina microalgae contain a plethora of nutrient and non-nutrient molecules provid- ing brain health benefits. Numerous in vivo evidence has provided support for the brain health potential of spirulina, highlighting antioxidant, anti-inflammatory, and neuroprotective mechanisms. Preliminary clinical studies have also suggested that spirulina can help to reduce mental fatigue, protect the vascular wall of brain vessels from endothelial damage and regulate internal pressure, Citation: Sorrenti, V.; Castagna, D.A.; thus contributing to the prevention and/or mitigating of cerebrovascular conditions. Furthermore, Fortinguerra, S.; Buriani, A.; the use of spirulina in malnourished children appears to ameliorate motor, language, and cognitive Scapagnini, G.; Willcox, D.C. skills, suggesting a reinforcing role in developmental mechanisms. -

Individual Sugars, Soluble, and Insoluble Dietary Fiber Contents of 70 High Consumption Foods
JOURNAL OF FOOD COMPOSITION AND ANALYSIS (2002) 15, 715–723 doi:10.1006/jfca.2002.1096 Available online at http://www.idealibrary.com on ORIGINAL ARTICLE Individual Sugars, Soluble, and Insoluble Dietary Fiber Contents of 70 High Consumption Foods Betty W. Li*,1, Karen W. Andrewsw,2, and Pamela R. Pehrssonw *Food Composition Laboratory, Beltsville Human Nutrition Research Center, ARS, USDA, U.S.A.;and wNutrient Data Laboratory, Beltsville Human Nutrition Research Center, ARS, USDA, U.S.A. Received April 30, 2002, and in revised form May 8, 2002 As part ofthe continuous efforts ofthe Nutrient Data Laboratory, Agricultural Research Service (ARS), USDA in updating and expanding the carbohydrate data in its database, foods were selected based on dietary fiber content and frequency of consumption. They were analyzed by a commercial testing laboratory under a USDA contract. Individual sugars, soluble and insoluble dietary fiber values of70 foods in six food groups were reported. Foods included 14 baked products, 10 cereal grains and pastas, 19 fruits, seven legumes, 10 cooked vegetables, and 10 raw vegetables. Except for cereal grains/pasta and legumes, most other foods contained fructose and glucose; sucrose was found in almost all except baked products, which were the most predominant source ofmaltose. Fruits contained the most total sugar and cereal, grains/pasta the least. Legumes contained the highest amount oftotal dietary fiber. All these commonly consumed foods, with the exception of cooked white rice, contained both soluble and insoluble dietary fiber. The percent ofsoluble and insoluble fiber varied across food groups, even within each group. Comparison ofdata fromthe commercial laboratory with those ofthe same foodanalyzed in the Food Composition Laboratory using different methods indicated there was good agreement between high-performance liquid chromatographic and gas chromato- graphic methods for the determination of individual sugars. -
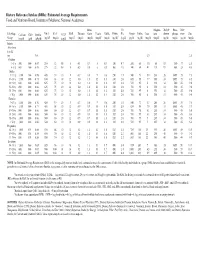
Dietary Reference Intakes (Dris): Estimated Average Requirements for Groups
Dietary Reference Intakes (DRIs): Estimated Average Requirements Food and Nutrition Board, Institute of Medicine, National Academies Ribo- Vit Magnes- Molyb- Phos- Sele- Life Stage Calcium CHO Protein Vit A Vit C Vit D Vit E Thiamin flavin Niacin Vit B6 Folate B12 Copper Iodine Iron ium denum phorus nium Zinc a b c d Group (mg/d) (g/d) (g/kg/d) (μg/d) (mg/d) (μg/d) (mg/d) (mg/d) (mg/d) (mg/d) (mg/d) (μg/d) (μg/d) (μg/d) (μg/d) (mg/d) (mg/d) (μg/d) (mg/d) (μg/d) (mg/d) Infants 0 to 6 mo 6 to 12 mo 1.0 6.9 2.5 Children 1–3 y 500 100 0.87 210 13 10 5 0.4 0.4 5 0.4 120 0.7 260 65 3.0 65 13 380 17 2.5 4–8 y 800 100 0.76 275 22 10 6 0.5 0.5 6 0.5 160 1.0 340 65 4.1 110 17 405 23 4.0 Males 9–13 y 1,100 100 0.76 445 39 10 9 0.7 0.8 9 0.8 250 1.5 540 73 5.9 200 26 1,055 35 7.0 14–18 y 1,100 100 0.73 630 63 10 12 1.0 1.1 12 1.1 330 2.0 685 95 7.7 340 33 1,055 45 8.5 19–30 y 800 100 0.66 625 75 10 12 1.0 1.1 12 1.1 320 2.0 700 95 6 330 34 580 45 9.4 31–50 y 800 100 0.66 625 75 10 12 1.0 1.1 12 1.1 320 2.0 700 95 6 350 34 580 45 9.4 51–70 y 800 100 0.66 625 75 10 12 1.0 1.1 12 1.4 320 2.0 700 95 6 350 34 580 45 9.4 > 70 y 1,000 100 0.66 625 75 10 12 1.0 1.1 12 1.4 320 2.0 700 95 6 350 34 580 45 9.4 Females 9–13 y 1,100 100 0.76 420 39 10 9 0.7 0.8 9 0.8 250 1.5 540 73 5.7 200 26 1,055 35 7.0 14–18 y 1,100 100 0.71 485 56 10 12 0.9 0.9 11 1.0 330 2.0 685 95 7.9 300 33 1,055 45 7.3 19–30 y 800 100 0.66 500 60 10 12 0.9 0.9 11 1.1 320 2.0 700 95 8.1 255 34 580 45 6.8 31–50 y 800 100 0.66 500 60 10 12 0.9 0.9 11 1.1 320 2.0 700 95 8.1 265 34 580 45 6.8