The Development of Fingolimod (FTY720, Gilenya)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Retinamide Increases Dihydroceramide and Synergizes with Dimethylsphingosine to Enhance Cancer Cell Killing
2967 N-(4-Hydroxyphenyl)retinamide increases dihydroceramide and synergizes with dimethylsphingosine to enhance cancer cell killing Hongtao Wang,1 Barry J. Maurer,1 Yong-Yu Liu,2 elevations in dihydroceramides (N-acylsphinganines), Elaine Wang,3 Jeremy C. Allegood,3 Samuel Kelly,3 but not desaturated ceramides, and large increases in Holly Symolon,3 Ying Liu,3 Alfred H. Merrill, Jr.,3 complex dihydrosphingolipids (dihydrosphingomyelins, Vale´rie Gouaze´-Andersson,4 Jing Yuan Yu,4 monohexosyldihydroceramides), sphinganine, and sphin- Armando E. Giuliano,4 and Myles C. Cabot4 ganine 1-phosphate. To test the hypothesis that elevation of sphinganine participates in the cytotoxicity of 4-HPR, 1Childrens Hospital Los Angeles, Keck School of Medicine, cells were treated with the sphingosine kinase inhibitor University of Southern California, Los Angeles, California; D-erythro-N,N-dimethylsphingosine (DMS), with and 2 College of Pharmacy, University of Louisiana at Monroe, without 4-HPR. After 24 h, the 4-HPR/DMS combination Monroe, Louisiana; 3School of Biology and Petit Institute of Bioengineering and Bioscience, Georgia Institute of Technology, caused a 9-fold increase in sphinganine that was sustained Atlanta, Georgia; and 4Gonda (Goldschmied) Research through +48 hours, decreased sphinganine 1-phosphate, Laboratories at the John Wayne Cancer Institute, and increased cytotoxicity. Increased dihydrosphingolipids Saint John’s Health Center, Santa Monica, California and sphinganine were also found in HL-60 leukemia cells and HT-29 colon cancer cells treated with 4-HPR. The Abstract 4-HPR/DMS combination elicited increased apoptosis in all three cell lines. We propose that a mechanism of N Fenretinide [ -(4-hydroxyphenyl)retinamide (4-HPR)] is 4-HPR–induced cytotoxicity involves increases in dihy- cytotoxic in many cancer cell types. -

The Change of Fingolimod Patient Profiles Over Time
Journal of Personalized Medicine Article The Change of Fingolimod Patient Profiles over Time: A Descriptive Analysis of Two Non-Interventional Studies PANGAEA and PANGAEA 2.0 Tjalf Ziemssen 1,* and Ulf Schulze-Topphoff 2 1 Zentrum für Klinische Neurowissenschaften, Universitätsklinikum Carl Gustav Carus, D-01307 Dresden, Germany 2 Novartis Pharma GmbH, D-90429 Nuremberg, Germany; [email protected] * Correspondence: [email protected] Abstract: (1) Background: Fingolimod (Gilenya®) was the first oral treatment for patients with relapsing-remitting multiple sclerosis (RRMS). Since its approval, the treatment landscape has changed enormously. (2) Methods: Data of PANGAEA and PANGAEA 2.0, two German real-world studies, were descriptively analysed for possible evolution of patient profiles and treatment behavior. Both are prospective, multi-center, non-interventional, long-term studies on fingolimod use in RRMS in real life. Data of 4229 PANGAEA patients (recruited 2011–2013) and 2441 PANGAEA 2.0 patients (recruited 2015–2018) were available. Baseline data included demographics, RRMS characteristics and disease severity. (3) Results: The mean age of PANGAEA and PANGAEA 2.0 patients was similar (38.8 vs. 39.2 years). Patients in PANGAEA 2.0 had shorter disease duration (7.1 vs. 8.2 years) and fewer relapses in the year before baseline (1.2 vs. 1.6). Disease severity at baseline estimated by Citation: Ziemssen, T.; EDSS and SDMT was lower in PANGAEA 2.0 patients compared to PANGAEA (EDSS difference Schulze-Topphoff, U. The Change of 1.0 points; SDMT difference 3.3 points). (4) Conclusions: The results hint at an influence of changes in Fingolimod Patient Profiles over the treatment guidelines and the label on fingolimod patients profiles over time. -

Medical Review(S) Clinical Review
CENTER FOR DRUG EVALUATION AND RESEARCH APPLICATION NUMBER: 22-527 MEDICAL REVIEW(S) CLINICAL REVIEW Application Type NDA Application Number 22-527 Priority or Standard P Submit Date 12-21-2009 Received Date 12-21-2009 PDUFA Goal Date 9-21-2010 Division / Office FDA/ODE1 Reviewer Name Heather D. Fitter Review Completion Date August 26, 2010 Established Name Fingolimod (Proposed) Trade Name Gilenya Therapeutic Class S1P receptor modulator Applicant Novartis Formulation(s) Oral tablets Dosing Regimen 0.5 mg Indication(s) To reduce the frequency of relapses and delay the progression of disability in relapsing MS Intended Population(s) Relapsing Multiple Sclerosis Template Version: March 6, 2009 Clinical Review Heather D. Fitter, M.D. NDA 22-527 Fingolimod Table of Contents 1 RECOMMENDATIONS/RISK BENEFIT ASSESSMENT......................................... 7 1.1 Recommendation on Regulatory Action ............................................................. 7 1.2 Risk Benefit Assessment.................................................................................... 8 2 INTRODUCTION AND REGULATORY BACKGROUND ........................................ 8 2.1 Product Information ............................................................................................ 9 2.2 Table of Currently Available Treatments for the Proposed Indication................. 9 2.3 Availability of Proposed Active Ingredient in the United States ........................ 12 2.4 Important Safety Issues with Consideration to Related Drugs......................... -

BƯỚC ĐẦU NGHIÊN CỨU CHI NẤM Isaria TẠI NÚI LANGBIAN THUỘC CAO NGUYÊN LÂM VIÊN
HỘI NGHỊ KHOA HỌC TOÀN QUỐC VỀ SINH THÁI VÀ TÀI NGUYÊN SINH VẬT LẦN THỨ 6 BƯỚC ĐẦU NGHIÊN CỨU CHI NẤM Isaria TẠI NÚI LANGBIAN THUỘC CAO NGUYÊN LÂM VIÊN ĐỖ THỊ THIÊN LÝ, PHẠM NỮ KIM HOÀNG, PHAN HỮU HÙNG, NGUYỄN VIẾT TRƯỜNG Viện Nghiên cứu Khoa học Tây Nguyên, Viện Hàn lâm Khoa học và Công nghệ Việt Nam LÊ HUYỀN ÁI THÚY Trường Đại học Mở Tp. Hồ Chí Minh TRƯƠNG BÌNH NGUYÊN Trường Đại học Đà Lạt Nấm ký sinh côn trùng (entomogenous fungi) là nhóm nấm rất phong phú về thành phần chi và loài. Một số chi được quan tâm bởi tính chất dược lý hay khả năng kiểm soát sinh học như Cordyceps, Beauveria và đặc biệt là chi nấm Isaria. Chi Isaria bao gồm các loài nấm ký sinh côn trùng phân bố khá rộng và thường gặp, dễ dàng thu thập. Isaria là giai đoạn sinh sản vô tính (giai đoạn hình thành bào tử đính) của chi Cordyceps thuộc lớp Ascomycetes. Các nghiên cứu trước đây đã xem xét Isaria farinose và Paecilomyces variotii là những loài thuộc chi Isaria Pers. ex Fr. do chúng có những đặc điểm tương tự nhau trong cấu trúc cuống sinh bào tử nên đã đề xuất Isaria nên được sử dụng cho tất cả các loài Paecilomyces [3][9][10]. Năm 1974, dựa trên sự phân nhánh của cuống bào tử đính, thể bình, cấu trúc thể bình và bào tử, Samson và cộng sự đã chia chi Paecilomyces thành hai phần là Paecilomyces sect. -
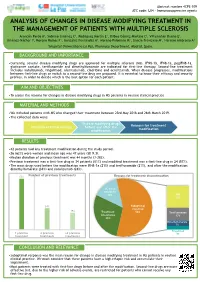
Analysis of Changes in Disease Modifying Treatment in The
Abstract number 4CPS-109 ATC code: L04 - Immunosuppressive agents ANALYSIS OF CHANGES IN DISEASE MODIFYING TREATMENT IN THE MANAGEMENT OF PATIENTS WITH MULTIPLE SCLEROSIS Arancón Pardo A1, Sobrino Jiménez C1, Rodríguez Martín E1, Bilbao Gómez-Martino C1, Villamañán Bueno E1, Jiménez-Nácher I1, Moreno Ramos F1, González Fernández A1, Moreno Palomino M1, García-Trevijano M1, Herrero Ambrosio A1 1Hospital Universitario La Paz, Pharmacy Department, Madrid, Spain. BACKGROUND AND IMPORTANCE •Currently, several disease modifying drugs are approved for multiple sclerosis (MS). IFNβ-1b, IFNβ-1a, pegIFNβ-1a, glatiramer acetate, teriflunomide and dimethylfumarate are indicated for first-line therapy. Second-line treatment includes natalizumab, fingolimod, alemtuzumab, cladribine and ocrelizumab. When disease progresses, modifications between first-line drugs or switch to a second-line drug are proposed. It is essential to know their efficacy and security profiles, in order to decide which is the best option for each patient. AIM AND OBJECTIVES •To assess the reasons for changes in disease modifying drugs in MS patients in routine clinical practice. MATERIAL AND METHODS •We included patients with MS who changed their treatment between 23rd May 2018 and 26th March 2019. •The collected data were: Disease modifying drugs Reasons for treatment Duration of initial therapy before and after the modification modification RESULTS •42 patients had any treatment modification during the study period. •26 (62%) were women and mean age was 47 years (SD 9.3). •Median duration of previous treatment was 44 months (3-282). •Previous treatment was a first-line drug in 34 patients (81%) and modified treatment was a first-line drug in 24 (57%). -

Patient Focused Disease State and Assistance Programs
Patient Focused Disease State and Assistance Programs Medication Medication Toll-free Brand (Generic) Website number Additional Resources Allergy/Asthma Xolair (omalizumab) xolair.com 1-866-4-XOLAIR lung.org Cardiovascular Pradaxa (dabigatran) pradaxa.com 877-481-5332 heart.org Praluent (alirocumab) praluent.com 844-PRALUENT thefhfoundation.org Repatha (evolocumab) repatha.com 844-REPATHA Tikosyn (dofetilide) tikosyn.com 800-879-3477 Crohn’s Disease Cimzia (certolizumab pegol) cimzia.com 866-4-CIMZIA crohnsandcolitis.com Humira (adalimumab) humira.com 800-4-HUMIRA crohnsforum.com Stelara (ustekinumab) stelarainfo.com 877-STELARA Dermatology Cosentyx (secukinumab) cosentyx.com 844-COSENTYX psoriasis.org Dupixent (dupilumab) dupixent.com 844-DUPIXENT nationaleczema.org Enbrel (etanercept) enbrel.com 888-4-ENBREL Humira (adalimumab) humira.com 800-4-HUMIRA Otezla (apremilast) otezla.com 844-4-OTEZLA Stelara (ustekinumab) stelarainfo.com 877-STELARA Taltz (ixekizumab) taltz.com 800-545-5979 Hematology Aranesp (darbepoetin alfa) aranesp.com 805-447-1000 chemocare.com Granix (filgrastim) granixrx.com 888-4-TEVARX hematology.org Jadenu (deferasirox) jadenu.com 888-282-7630 Neulasta (pegfilgrastim) neulasta.com 800-77-AMGEN Neupogen (filgrastim) neupogen.com 800-77-AMGEN Nivestym (filgrastim) nivestym.com 800-879-3477 Zarxio (filgrastim) zarxio.com 800-525-8747 Zytiga (abiraterone) zytiga.com 800-JANSSEN Hepatitis B Baraclude (entecavir) baraclude.com 800-321-1335 cdc.gov Viread (tenofovir disoproxil viread.com 800-GILEAD-5 hepb.org fumarate) -

COMPARISON of the WHO ATC CLASSIFICATION & Ephmra/Intellus Worldwide ANATOMICAL CLASSIFICATION
COMPARISON OF THE WHO ATC CLASSIFICATION & EphMRA/Intellus Worldwide ANATOMICAL CLASSIFICATION: VERSION June 2019 2 Comparison of the WHO ATC Classification and EphMRA / Intellus Worldwide Anatomical Classification The following booklet is designed to improve the understanding of the two classification systems. The development of the two systems had previously taken place separately. EphMRA and WHO are now working together to ensure that there is a convergence of the 2 systems rather than a divergence. In order to better understand the two classification systems, we should pay attention to the way in which substances/products are classified. WHO mainly classifies substances according to the therapeutic or pharmaceutical aspects and in one class only (particular formulations or strengths can be given separate codes, e.g. clonidine in C02A as antihypertensive agent, N02C as anti-migraine product and S01E as ophthalmic product). EphMRA classifies products, mainly according to their indications and use. Therefore, it is possible to find the same compound in several classes, depending on the product, e.g., NAPROXEN tablets can be classified in M1A (antirheumatic), N2B (analgesic) and G2C if indicated for gynaecological conditions only. The purposes of classification are also different: The main purpose of the WHO classification is for international drug utilisation research and for adverse drug reaction monitoring. This classification is recommended by the WHO for use in international drug utilisation research. The EphMRA/Intellus Worldwide classification has a primary objective to satisfy the marketing needs of the pharmaceutical companies. Therefore, a direct comparison is sometimes difficult due to the different nature and purpose of the two systems. -

Neurological S1P Signaling As an Emerging Mechanism of Action of Oral FTY720 (Fingolimod) in Multiple Sclerosis
Arch Pharm Res Vol 33, No 10, 1567-1574, 2010 DOI 10.1007/s12272-010-1008-5 REVIEW Neurological S1P Signaling as an Emerging Mechanism of Action of Oral FTY720 (Fingolimod) in Multiple Sclerosis Chang Wook Lee1, Ji Woong Choi2, and Jerold Chun1 1Department of Molecular Biology, Dorris Neuroscience Center, The Scripps Research Institute, La Jolla, California 92037, USA and 2Department of Pharmacology, Division of Basic Medicine and Science, Gachon University of Medi- cine and Science, Incheon 406-799, Korea (Received August 15, 2010/Revised August 23, 2010/Accepted August 24, 2010) FTY720 (fingolimod, Novartis) is a promising investigational drug for relapsing forms of multi- ple sclerosis (MS), an autoimmune and neurodegenerative disorder of the central nervous sys- tem. It is currently under FDA review in the United States, and could represent the first approved oral treatment for MS. Extensive, ongoing clinical trials in Phase II/III have sup- ported both the efficacy and safety of FTY720. FTY720 itself is not bioactive, but when phospho- rylated (FTY720-P) by sphingosine kinase 2, it becomes active through modulation of 4 of the 5 known G protein-coupled sphingosine 1-phosphate (S1P) receptors. The mechanism of action (MOA) is thought to be immunological, where FTY720 alters lymphocyte trafficking via S1P1. However, MOA for FTY720 in MS may also involve a direct, neurological action within the cen- tral nervous system in view of documented S1P receptor-mediated signaling influences in the brain, and this review considers observations that support an emerging neurological MOA. Key words: FTY720, Fingolimod, Sphingosine 1-phosphate receptors, Multiple sclerosis, Cen- tral nervous system INTRODUCTION factors that initiate MS are still unknown. -

CPY Document
54 Transactions British Mycological Society 55 (i i) GALLØE, o. Natural History of the Danish Lichens, i (1927), II (1929). (i 2) HARMAND, i. Guide élémentaire du Lichénologue accompagné de nombreuses espèces typiques en nature (i 904). NOTES ON ENTOMOGENOUS FUNGI (13) HUE, A. M. Lichens. Deuxième Expédition Antarctique Française (1908- I9IO), commandée par Ie Dr Jean Charcot, Paris (1915). By T. PETeR (14) KRABBE, G. Entwickelungsgeschichte und Morphologie der polymorphen Flechten- gattung Cladonia (i 89 i ). (With 4 Text-figures) (i 5) NEUBNER, E. "Untersuchungen über den Thallus und die Fruchtanfånge der Calycieen." Wiss. Beil. des iv. Jahresber. k. Gymnas. z. Plauen i.IV. (i893). I. BEA UVERIA PETELOTI Vincens (16) NIENBURG, W. "Ueber die Beziehungen zwischen den Algen und Hyphen im Flechtenthallus." Zeitsch.j. Bot. ix (1917), 529-543. BEAUVERIA PETELOTI Vincens was described by Vincens in Bull. Soc. (17) PAULSON, R. "The sporulation ofgonidia in the thallus of Everniaprunastri Ach." Trans. Brit. Myc. Soc. VII (1922),44-46. Bot. France, Sér. 4, xv (1915), 132-144, from three collections from (18) PAULSON, R. and HASTINGS, S. "The relation between the alga and fungus oft fBrazil, one on the wasp, Polybia chrysothorax, another on the wasp, a lichen." Journ. Linn. Soc. XLIV (1920), 497-506. i ¡polystes canadensis, and the third on bees, the fungus being so different (19) Natuiforsch.SCHWNDENER, S. "Ueber Gesell. den Bau und in das WachsthumZürich des (1860). Flechtenthallus."! ;i Hn appearance in the three cases, that, as remarked by Vincens, one would consider them three different species, were it not that a study (20) - "Ueber die Apothecia primitus aperta und die Entwicklung der Apothecieni im Allgemeinen." Flora, XLVII (1864),321-332. -

The Real-World Patient Experience of Fingolimod and Dimethyl Fumarate
Wicks et al. BMC Res Notes (2016) 9:434 DOI 10.1186/s13104-016-2243-8 BMC Research Notes RESEARCH ARTICLE Open Access The real‑world patient experience of fingolimod and dimethyl fumarate for multiple sclerosis Paul Wicks1* , Lawrence Rasouliyan2, Bo Katic1, Beenish Nafees3, Emuella Flood3 and Rahul Sasané4 Abstract Background: Oral disease-modifying therapies offer equivalent or superior efficacy and greater convenience versus injectable options. Objectives: To compare patient-reported experiences of fingolimod and dimethyl fumarate. Methods: Adult relapsing-remitting multiple sclerosis patients treated with fingolimod or dimethyl fumarate were recruited from an online patient community and completed an online survey about treatment side effects, discon- tinuation, and satisfaction. Results: 281 patients in four groups completed the survey: currently receiving fingolimod (CF, N 61), currently receiving dimethyl fumarate (CDMF, N 129), discontinued fingolimod (DF, N 32) and discontinued= dimethyl fuma- rate (DDMF, N 59). Reasons for treatment= switch were to take oral treatment =(CF: 63.3 %, CDMF: 61.8 %), side effects of prior medication= (CF: 67.3 %, CDMF: 44.1 %) and lack of effectiveness of prior medication (CF: 38.8 %, CDMF: 31.4 %). Main reasons for discontinuation were side effects (DF: 46.9 %, DDMF: 67.8 %) and lack of effectiveness (DF: 25.0 %, DDMF: 15.3 %). CDMF patients had an increased risk of abdominal pain, flushing, diarrhea, and nausea. Treatment satisfaction was highest among CF patients followed by CDMF, DF, and then DDMF patients. Conclusions: Discontinuation was driven by experience of side effects. Patients currently taking dimethyl fumarate were more likely to experience a side effect versus patients currently taking fingolimod. -

Fingolimod (Gilenya)
Fingolimod (Gilenya) This factsheet is about fingolimod, a Whether you’ll be offered this or any other DMT disease modifying therapy (DMT) for depends on whether you qualify for it based relapsing multiple sclerosis (MS). on guidelines used by your neurologist. These come from NICE and the Association of British At the end of this factsheet you’ll find out where Neurologists and are based on a drug’s Europe- you can get more information on this drug, other wide licence. drugs for MS and the benefits of early treatment. In England there are also rules from NHS England about who can have the different DMTs and when. This factsheet doesn’t cover everything about Scotland, Wales and Northern Ireland also have this drug and shouldn’t be used in place of their own guidelines for many DMTs. advice from your MS specialist team. For more information speak to them and read the online information from the drug’s makers (see the Whether you can have a drug also depends on section More information and support). if the NHS where you live will pay for it. NHS England guidelines on this tend to follow what What is fingolimod? NICE says. Fingolimod is a drug that was first given a licence These people can have fingolimod: to be used against relapsing MS in the UK in 2011. In England and Northern Ireland: In 2012 the National Institute for Health and Care Excellence (NICE) gave the go ahead for it to be • People with ‘highly active relapsing remitting used on the NHS. -

Fingolimod (FTY720): Discovery and Development of an Oral Drug to Treat Multiple Sclerosis
REVIEWS CASE HISTORY Fingolimod (FTY720): discovery and development of an oral drug to treat multiple sclerosis Volker Brinkmann*, Andreas Billich*, Thomas Baumruker*, Peter Heining‡, Robert Schmouder§, Gordon Francis||, Shreeram Aradhye¶ and Pascale Burtin# Abstract | The discovery of fingolimod (FTY720/Gilenya; Novartis), an orally active immunomodulatory drug, has opened up new approaches to the treatment of multiple sclerosis, the most common inflammatory disorder of the central nervous system. Elucidation of the effects of fingolimod — mediated by the modulation of sphingosine 1‑phosphate (S1P) receptors — has indicated that its therapeutic activity could be due to regulation of the migration of selected lymphocyte subsets into the central nervous system and direct effects on neural cells, particularly astrocytes. An improved understanding of the biology of S1P receptors has also been gained. This article describes the discovery and development of fingolimod, which was approved by the US Food and Drug Administration in September 2010 as a first‑line treatment for relapsing forms of multiple sclerosis, thereby becoming the first oral disease‑modifying therapy to be approved for multiple sclerosis in the United States. Demyelination Multiple sclerosis (MS) is a chronic autoimmune disorder Treatment strategies for MS usually involve the man- Damage of the myelin sheath of the central nervous system (CNS) that is characterized agement of symptoms and the use of disease-modifying of axons. A demyelinating by inflammation leading to astrogliosis, demyelination, drugs to reduce the frequency of relapses and to slow disease is any disease of and loss of oligodendrocytes and neurons1. MS is the the progression of disability. Established first-line the nervous system in which the myelin sheath is damaged.