Beyond the Fish-Daphnia Paradigm: Testing the Potential For
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Insetos Do Brasil
COSTA LIMA INSETOS DO BRASIL 2.º TOMO HEMÍPTEROS ESCOLA NACIONAL DE AGRONOMIA SÉRIE DIDÁTICA N.º 3 - 1940 INSETOS DO BRASIL 2.º TOMO HEMÍPTEROS A. DA COSTA LIMA Professor Catedrático de Entomologia Agrícola da Escola Nacional de Agronomia Ex-Chefe de Laboratório do Instituto Oswaldo Cruz INSETOS DO BRASIL 2.º TOMO CAPÍTULO XXII HEMÍPTEROS ESCOLA NACIONAL DE AGRONOMIA SÉRIE DIDÁTICA N.º 3 - 1940 CONTEUDO CAPÍTULO XXII PÁGINA Ordem HEMÍPTERA ................................................................................................................................................ 3 Superfamília SCUTELLEROIDEA ............................................................................................................ 42 Superfamília COREOIDEA ............................................................................................................................... 79 Super família LYGAEOIDEA ................................................................................................................................. 97 Superfamília THAUMASTOTHERIOIDEA ............................................................................................... 124 Superfamília ARADOIDEA ................................................................................................................................... 125 Superfamília TINGITOIDEA .................................................................................................................................... 132 Superfamília REDUVIOIDEA ........................................................................................................................... -

RECORDS of the HAWAII BIOLOGICAL SURVEY for 1995 Part 2: Notes1
RECORDS OF THE HAWAII BIOLOGICAL SURVEY FOR 1995 Part 2: Notes1 This is the second of two parts to the Records of the Hawaii Biological Survey for 1995 and contains the notes on Hawaiian species of plants and animals including new state and island records, range extensions, and other information. Larger, more compre- hensive treatments and papers describing new taxa are treated in the first part of this Records [Bishop Museum Occasional Papers 45]. New Hawaiian Pest Plant Records for 1995 PATRICK CONANT (Hawaii Dept. of Agriculture, Plant Pest Control Branch, 1428 S King St, Honolulu, HI 96814) Fabaceae Ulex europaeus L. New island record On 6 October 1995, Hawaii Department of Land and Natural Resources, Division of Forestry and Wildlife employee C. Joao submitted an unusual plant he found while work- ing in the Molokai Forest Reserve. The plant was identified as U. europaeus and con- firmed by a Hawaii Department of Agriculture (HDOA) nox-A survey of the site on 9 October revealed an infestation of ca. 19 m2 at about 457 m elevation in the Kamiloa Distr., ca. 6.2 km above Kamehameha Highway. Distribution in Wagner et al. (1990, Manual of the flowering plants of Hawai‘i, p. 716) listed as Maui and Hawaii. Material examined: MOLOKAI: Molokai Forest Reserve, 4 Dec 1995, Guy Nagai s.n. (BISH). Melastomataceae Miconia calvescens DC. New island record, range extensions On 11 October, a student submitted a leaf specimen from the Wailua Houselots area on Kauai to PPC technician A. Bell, who had the specimen confirmed by David Lorence of the National Tropical Botanical Garden as being M. -

Hundreds of Species of Aquatic Macroinvertebrates Live in Illinois In
Illinois A B aquatic sowbug Asellus sp. Photograph © Paul P.Tinerella AAqquuaattiicc mayfly A. adult Hexagenia sp.; B. nymph Isonychia sp. MMaaccrrooiinnvveerrtteebbrraatteess Photographs © Michael R. Jeffords northern clearwater crayfish Orconectes propinquus Photograph © Michael R. Jeffords ruby spot damselfly Hetaerina americana Photograph © Michael R. Jeffords aquatic snail Pleurocera acutum Photograph © Jochen Gerber,The Field Museum of Natural History predaceous diving beetle Dytiscus circumcinctus Photograph © Paul P.Tinerella monkeyface mussel Quadrula metanevra common skimmer dragonfly - nymph Libellula sp. Photograph © Kevin S. Cummings Photograph © Paul P.Tinerella water scavenger beetle Hydrochara sp. Photograph © Steve J.Taylor devil crayfish Cambarus diogenes A B Photograph © ChristopherTaylor dobsonfly Corydalus sp. A. larva; B. adult Photographs © Michael R. Jeffords common darner dragonfly - nymph Aeshna sp. Photograph © Paul P.Tinerella giant water bug Belostoma lutarium Photograph © Paul P.Tinerella aquatic worm Slavina appendiculata Photograph © Mark J. Wetzel water boatman Trichocorixa calva Photograph © Paul P.Tinerella aquatic mite Order Prostigmata Photograph © Michael R. Jeffords backswimmer Notonecta irrorata Photograph © Paul P.Tinerella leech - adult and young Class Hirudinea pygmy backswimmer Neoplea striola mosquito - larva Toxorhynchites sp. fishing spider Dolomedes sp. Photograph © William N. Roston Photograph © Paul P.Tinerella Photograph © Michael R. Jeffords Photograph © Paul P.Tinerella Species List Species are not shown in proportion to actual size. undreds of species of aquatic macroinvertebrates live in Illinois in a Kingdom Animalia Hvariety of habitats. Some of the habitats have flowing water while Phylum Annelida Class Clitellata Family Naididae aquatic worm Slavina appendiculata This poster was made possible by: others contain still water. In order to survive in water, these organisms Class Hirudinea leech must be able to breathe, find food, protect themselves, move and reproduce. -

Underwater Breathing: the Mechanics of Plastron Respiration
J. Fluid Mech. (2008), vol. 608, pp. 275–296. c 2008 Cambridge University Press 275 doi:10.1017/S0022112008002048 Printed in the United Kingdom Underwater breathing: the mechanics of plastron respiration M. R. FLYNN† AND J O H N W. M. B U S H Department of Mathematics, Massachusetts Institute of Technology, 77 Massachusetts Avenue, Cambridge, MA 02139-4307, USA (Received 11 July 2007 and in revised form 10 April 2008) The rough, hairy surfaces of many insects and spiders serve to render them water-repellent; consequently, when submerged, many are able to survive by virtue of a thin air layer trapped along their exteriors. The diffusion of dissolved oxygen from the ambient water may allow this layer to function as a respiratory bubble or ‘plastron’, and so enable certain species to remain underwater indefinitely. Main- tenance of the plastron requires that the curvature pressure balance the pressure difference between the plastron and ambient. Moreover, viable plastrons must be of sufficient area to accommodate the interfacial exchange of O2 and CO2 necessary to meet metabolic demands. By coupling the bubble mechanics, surface and gas-phase chemistry, we enumerate criteria for plastron viability and thereby deduce the range of environmental conditions and dive depths over which plastron breathers can survive. The influence of an external flow on plastron breathing is also examined. Dynamic pressure may become significant for respiration in fast-flowing, shallow and well-aerated streams. Moreover, flow effects are generally significant because they sharpen chemical gradients and so enhance mass transfer across the plastron interface. Modelling this process provides a rationale for the ventilation movements documented in the biology literature, whereby arthropods enhance plastron respiration by flapping their limbs or antennae. -

The Semiaquatic Hemiptera of Minnesota (Hemiptera: Heteroptera) Donald V
The Semiaquatic Hemiptera of Minnesota (Hemiptera: Heteroptera) Donald V. Bennett Edwin F. Cook Technical Bulletin 332-1981 Agricultural Experiment Station University of Minnesota St. Paul, Minnesota 55108 CONTENTS PAGE Introduction ...................................3 Key to Adults of Nearctic Families of Semiaquatic Hemiptera ................... 6 Family Saldidae-Shore Bugs ............... 7 Family Mesoveliidae-Water Treaders .......18 Family Hebridae-Velvet Water Bugs .......20 Family Hydrometridae-Marsh Treaders, Water Measurers ...22 Family Veliidae-Small Water striders, Rime bugs ................24 Family Gerridae-Water striders, Pond skaters, Wherry men .....29 Family Ochteridae-Velvety Shore Bugs ....35 Family Gelastocoridae-Toad Bugs ..........36 Literature Cited ..............................37 Figures ......................................44 Maps .........................................55 Index to Scientific Names ....................59 Acknowledgement Sincere appreciation is expressed to the following individuals: R. T. Schuh, for being extremely helpful in reviewing the section on Saldidae, lending specimens, and allowing use of his illustrations of Saldidae; C. L. Smith for reading the section on Veliidae, checking identifications, and advising on problems in the taxon omy ofthe Veliidae; D. M. Calabrese, for reviewing the section on the Gerridae and making helpful sugges tions; J. T. Polhemus, for advising on taxonomic prob lems and checking identifications for several families; C. W. Schaefer, for providing advice and editorial com ment; Y. A. Popov, for sending a copy ofhis book on the Nepomorpha; and M. C. Parsons, for supplying its English translation. The University of Minnesota, including the Agricultural Experi ment Station, is committed to the policy that all persons shall have equal access to its programs, facilities, and employment without regard to race, creed, color, sex, national origin, or handicap. The information given in this publication is for educational purposes only. -

Biological Diversity, Ecological Health and Condition of Aquatic Assemblages at National Wildlife Refuges in Southern Indiana, USA
Biodiversity Data Journal 3: e4300 doi: 10.3897/BDJ.3.e4300 Taxonomic Paper Biological Diversity, Ecological Health and Condition of Aquatic Assemblages at National Wildlife Refuges in Southern Indiana, USA Thomas P. Simon†, Charles C. Morris‡, Joseph R. Robb§, William McCoy | † Indiana University, Bloomington, IN 46403, United States of America ‡ US National Park Service, Indiana Dunes National Lakeshore, Porter, IN 47468, United States of America § US Fish and Wildlife Service, Big Oaks National Wildlife Refuge, Madison, IN 47250, United States of America | US Fish and Wildlife Service, Patoka River National Wildlife Refuge, Oakland City, IN 47660, United States of America Corresponding author: Thomas P. Simon ([email protected]) Academic editor: Benjamin Price Received: 08 Dec 2014 | Accepted: 09 Jan 2015 | Published: 12 Jan 2015 Citation: Simon T, Morris C, Robb J, McCoy W (2015) Biological Diversity, Ecological Health and Condition of Aquatic Assemblages at National Wildlife Refuges in Southern Indiana, USA. Biodiversity Data Journal 3: e4300. doi: 10.3897/BDJ.3.e4300 Abstract The National Wildlife Refuge system is a vital resource for the protection and conservation of biodiversity and biological integrity in the United States. Surveys were conducted to determine the spatial and temporal patterns of fish, macroinvertebrate, and crayfish populations in two watersheds that encompass three refuges in southern Indiana. The Patoka River National Wildlife Refuge had the highest number of aquatic species with 355 macroinvertebrate taxa, six crayfish species, and 82 fish species, while the Big Oaks National Wildlife Refuge had 163 macroinvertebrate taxa, seven crayfish species, and 37 fish species. The Muscatatuck National Wildlife Refuge had the lowest diversity of macroinvertebrates with 96 taxa and six crayfish species, while possessing the second highest fish species richness with 51 species. -

Insect Classification Standards 2020
RECOMMENDED INSECT CLASSIFICATION FOR UGA ENTOMOLOGY CLASSES (2020) In an effort to standardize the hexapod classification systems being taught to our students by our faculty in multiple courses across three UGA campuses, I recommend that the Entomology Department adopts the basic system presented in the following textbook: Triplehorn, C.A. and N.F. Johnson. 2005. Borror and DeLong’s Introduction to the Study of Insects. 7th ed. Thomson Brooks/Cole, Belmont CA, 864 pp. This book was chosen for a variety of reasons. It is widely used in the U.S. as the textbook for Insect Taxonomy classes, including our class at UGA. It focuses on North American taxa. The authors were cautious, presenting changes only after they have been widely accepted by the taxonomic community. Below is an annotated summary of the T&J (2005) classification. Some of the more familiar taxa above the ordinal level are given in caps. Some of the more important and familiar suborders and families are indented and listed beneath each order. Note that this is neither an exhaustive nor representative list of suborders and families. It was provided simply to clarify which taxa are impacted by some of more important classification changes. Please consult T&J (2005) for information about taxa that are not listed below. Unfortunately, T&J (2005) is now badly outdated with respect to some significant classification changes. Therefore, in the classification standard provided below, some well corroborated and broadly accepted updates have been made to their classification scheme. Feel free to contact me if you have any questions about this classification. -

Final Scoping Report
Integrated Environmental Authorisations for the Proposed Expansion of the Nomalanga Estate, KwaZulu-Natal Province Final Scoping Report Project Number: NOM5486 Prepared for: Nomalanga Property Holdings (Pty) Ltd January 2019 _______________________________________________________________________________________ Digby Wells and Associates (South Africa) (Pty) Ltd Co. Reg. No. 2010/008577/07. Turnberry Office Park, 48 Grosvenor Road, Bryanston, 2191. Private Bag X10046, Randburg, 2125, South Africa Tel: +27 11 789 9495, Fax: +27 11 069 6801, [email protected], www.digbywells.com _______________________________________________________________________________________ Directors: GE Trusler (C.E.O), GB Beringer, LF Koeslag, J Leaver (Chairman)*, NA Mehlomakulu*, DJ Otto *Non-Executive _______________________________________________________________________________________ Final Scoping Report Integrated Environmental Authorisations for the Proposed Expansion of the Nomalanga Estate, KwaZulu-Natal Province NOM5486 This document has been prepared by Digby Wells Environmental. Report Type: Final Scoping Report Integrated Environmental Authorisations for the Proposed Project Name: Expansion of the Nomalanga Estate, KwaZulu-Natal Province Project Code: NOM5486 Name Responsibility Signature Date Manisha Nana Report Compilation October 2018 Sanusha Govender Report Compilation January 2019 Barbara Wessels Report Review October 2018 This report is provided solely for the purposes set out in it and may not, in whole or in part, be used for any other -

Aquatic Macroinvertebrates Section a Aquatic Macroinvertebrates (Exclusive of Mosquitoes)
I LLINOI S UNIVERSITY OF ILLINOIS AT URBANA-CHAMPAIGN PRODUCTION NOTE University of Illinois at Urbana-Champaign Library Large-scale Digitization Project, 2007. \oc iatural History Survey. Library iiAOs (ClSCi;; ILLINOIS - NATURAL HISTORY Ai . .ý . - I-w. Iv mk U16 OL SURVEY CHAPTER 9 AQUATIC MACROINVERTEBRATES SECTION A AQUATIC MACROINVERTEBRATES (EXCLUSIVE OF MOSQUITOES) Final Report October, 1985 Section of Faunistic Surveys and Insect Identification Technical Report by Allison R. Brigham, Lawrence M. Page, John D. Unzicker Mark J. Wetzel, Warren U. Brigham, Donald W. Webb, and Liane Suloway Prepared for Wetlands Research, Inc. 53 West Jackson Boulevard Chicago, IL 60604 Arjpp, Section of Faunistic Surveys and Insect Identification Technical Report 1985 (6) 6'Wa- CHAPTER 9 AQUATIC MACROINVERTEBRATES SECTION A AQUATIC MACROINVERTEBRATES (EXCLUSIVE OF MOSQUITOES) Allison R. Brigham, Lawrence M. Page, John D. Unzicker Mark J. Wetzel, Warren U. Brigham, Donald W. Webb, and Liane Suloway INTRODUCTION Aquatic macroinvertebrates are primary and secondary level consumers that play an important role in transferring energy through the different trophic levels of the food chains of aquatic ecosystems. These animals feed upon submerged and emergent macrophytes, plankton, and organic material suspended in the water column. Burrowing and feeding activities aid in the decomposition of plant and animal matter and the eventual recycling of nutrients. In addition, these organisms prey upon each other and serve as food for fishes, certain birds, and other animals. In general, aquatic macroinvertebrates have not been systematically surveyed in Illinois, and rarely have individual species been studied ecologically. This is due, in part, to the inconspicuous nature of most freshwater inverte- brates and the many taxonomic problems which preclude distributional, ecologi- cal, and other studies. -

The Water Bugs (Hemiptera; Heteroptera) from the Western Thong Pha Phum Research Project Area, Kanchanaburi Province, Thailand
รายงานการวิจัยในโครงการ 38-51 ชุดโครงการทองผาภูมิตะวันตก The Water Bugs (Hemiptera; Heteroptera) from the Western Thong Pha Phum Research Project Area, Kanchanaburi Province, Thailand Chariya Lekprayoon*, Marut Fuangarworn and Ezra Mongkolchaichana Chulalongkorn University, Bangkok *[email protected] Abstracts: Water bugs belong to the order Hemiptera, suborder Heteroptera which contains two kinds of members; semiaquatic (Gerromorpha), and true water bugs (Nepomorpha). They play a major role as biological control agents, and ecologically as food for higher trophic levels (birds and fish). This study is aimed at ascertaining the basic biodiversity and distribution, as well as biological and ecological based data, of water bugs in Thailand and to this aim this part the research was conducted at 4 locations of lotic habitats during May 2002 to April 2003 and at 4 wetland locations during May 2005 to June 2006, in the western Thong Pha Phum research project area. Data on the physical factors of each location were recorded at the time of collection of water bugs. Fifty-six species, from 49 genera and 14 families, were identified but this is an underestimate of the true biodiversity with and more than 16 different morphospecies likely to represent but true different species still in the process of identification. Timasius chesadai Chen, Nieser and Lekprayoon, 2006 (Hebridae) was found and described as a new species and the first record from Thailand. To aid future researchers, a key to families of Heteroptera within the Thong Pha Phum area of Thailand was prepared and is presented along with summary biological and ecological information at the family level. This report on species diversity of water bugs suggests that at least 72 species are expected to have been found from the west Thong Pha Phum area, a small part of Thailand. -
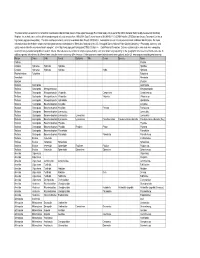
This Table Contains a Taxonomic List of Benthic Invertebrates Collected from Streams in the Upper Mississippi River Basin Study
This table contains a taxonomic list of benthic invertebrates collected from streams in the Upper Mississippi River Basin study unit as part of the USGS National Water Quality Assessemnt (NAWQA) Program. Invertebrates were collected from woody snags in selected streams from 1996-2004. Data Retreival occurred 26-JAN-06 11.10.25 AM from the USGS data warehouse (Taxonomic List Invert http://water.usgs.gov/nawqa/data). The data warehouse currently contains invertebrate data through 09/30/2002. Invertebrate taxa can include provisional and conditional identifications. For more information about invertebrate sample processing and taxonomic standards see, "Methods of analysis by the U.S. Geological Survey National Water Quality Laboratory -- Processing, taxonomy, and quality control of benthic macroinvertebrate samples", at << http://nwql.usgs.gov/Public/pubs/OFR00-212.html >>. Data Retrieval Precaution: Extreme caution must be exercised when comparing taxonomic lists generated using different search criteria. This is because the number of samples represented by each taxa list will vary depending on the geographic criteria selected for the retrievals. In addition, species lists retrieved at different times using the same criteria may differ because: (1) the taxonomic nomenclature (names) were updated, and/or (2) new samples containing new taxa may Phylum Class Order Family Subfamily Tribe Genus Species Taxon Porifera Porifera Cnidaria Hydrozoa Hydroida Hydridae Hydridae Cnidaria Hydrozoa Hydroida Hydridae Hydra Hydra sp. Platyhelminthes Turbellaria Turbellaria Nematoda Nematoda Bryozoa Bryozoa Mollusca Gastropoda Gastropoda Mollusca Gastropoda Mesogastropoda Mesogastropoda Mollusca Gastropoda Mesogastropoda Viviparidae Campeloma Campeloma sp. Mollusca Gastropoda Mesogastropoda Viviparidae Viviparus Viviparus sp. Mollusca Gastropoda Mesogastropoda Hydrobiidae Hydrobiidae Mollusca Gastropoda Basommatophora Ancylidae Ancylidae Mollusca Gastropoda Basommatophora Ancylidae Ferrissia Ferrissia sp. -

Great Lakes Entomologist the Grea T Lakes E N Omo L O G Is T Published by the Michigan Entomological Society Vol
The Great Lakes Entomologist THE GREA Published by the Michigan Entomological Society Vol. 45, Nos. 3 & 4 Fall/Winter 2012 Volume 45 Nos. 3 & 4 ISSN 0090-0222 T LAKES Table of Contents THE Scholar, Teacher, and Mentor: A Tribute to Dr. J. E. McPherson ..............................................i E N GREAT LAKES Dr. J. E. McPherson, Educator and Researcher Extraordinaire: Biographical Sketch and T List of Publications OMO Thomas J. Henry ..................................................................................................111 J.E. McPherson – A Career of Exemplary Service and Contributions to the Entomological ENTOMOLOGIST Society of America L O George G. Kennedy .............................................................................................124 G Mcphersonarcys, a New Genus for Pentatoma aequalis Say (Heteroptera: Pentatomidae) IS Donald B. Thomas ................................................................................................127 T The Stink Bugs (Hemiptera: Heteroptera: Pentatomidae) of Missouri Robert W. Sites, Kristin B. Simpson, and Diane L. Wood ............................................134 Tymbal Morphology and Co-occurrence of Spartina Sap-feeding Insects (Hemiptera: Auchenorrhyncha) Stephen W. Wilson ...............................................................................................164 Pentatomoidea (Hemiptera: Pentatomidae, Scutelleridae) Associated with the Dioecious Shrub Florida Rosemary, Ceratiola ericoides (Ericaceae) A. G. Wheeler, Jr. .................................................................................................183