Ecology and Management of Kudzu Bug (Hemiptera: Plataspidae) in Southeastern Soybeans
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Improved Conservation Plant Materials Released by NRCS and Cooperators Through December 2014
Natural Resources Conservation Service Improved Conservation Plant Materials Released by Plant Materials Program NRCS and Cooperators through December 2014 Page intentionally left blank. Natural Resources Conservation Service Plant Materials Program Improved Conservation Plant Materials Released by NRCS and Cooperators Through December 2014 Norman A. Berg Plant Materials Center 8791 Beaver Dam Road Building 509, BARC-East Beltsville, Maryland 20705 U.S.A. Phone: (301) 504-8175 prepared by: Julie A. DePue Data Manager/Secretary [email protected] John M. Englert Plant Materials Program Leader [email protected] January 2015 Visit our Website: http://Plant-Materials.nrcs.usda.gov TABLE OF CONTENTS Topics Page Introduction ...........................................................................................................................................................1 Types of Plant Materials Releases ........................................................................................................................2 Sources of Plant Materials ....................................................................................................................................3 NRCS Conservation Plants Released in 2013 and 2014 .......................................................................................4 Complete Listing of Conservation Plants Released through December 2014 ......................................................6 Grasses ......................................................................................................................................................8 -
Behind the Scenes, I Have Been Watching the Bowerbird Record
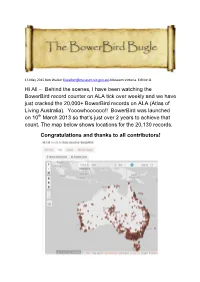
15 May 2015 Ken Walker ([email protected]) Museum Victoria. Edition 8. Hi All – Behind the scenes, I have been watching the BowerBird record counter on ALA tick over weekly and we have just cracked the 20,000+ BowerBird records on ALA (Atlas of Living Australia). Yooowhoooooo!! BowerBird was launched on 10th March 2013 so that’s just over 2 years to achieve that count. The map below shows locations for the 20,130 records. Congratulations and thanks to all contributors! From a purely funding analysis point of view, the initial ALA grant to develop BowerBird was $350,000 which at 20,130 records is currently returning per record at a cost of $17.38 and that per record cost will only get lower as more BowerBird records are added and uploaded. For some species on ALA, BowerBird provides the only distributional species data points: For other species on ALA, BowerBird provides the only species image for species on ALA: From a Biosecurity point of view of tracking exotic species, BowerBird has supplied all records post 2007 for the exotic South African Carder bee. All of the blue dots represent BowerBird records. Other than the great biodiversity results that BowerBird delivers through ALA, there are a myriad of other intangible benefits that come from the BowerBird website. Intangibles such as the member’s conversations, identification discussions, the friendships, the biological statements and the generated innate knowledge. One particular “intangible”, I would like to tell you about here. The PBCRC (Plant Biosecurity, Cooperative Research Centre) has a theme called “Building Resilience through Remote Indigenous Engagement”. -

Peanut Stunt Virus Infecting Perennial Peanuts in Florida and Georgia1 Carlye Baker2, Ann Blount3, and Ken Quesenberry4
Plant Pathology Circular No. 395 Fla. Dept. of Agric. & Consumer Serv. ____________________________________________________________________________________July/August 1999 Division of Plant Industry Peanut Stunt Virus Infecting Perennial Peanuts in Florida and Georgia1 Carlye Baker2, Ann Blount3, and Ken Quesenberry4 INTRODUCTION: Peanut stunt virus (PSV) has been reported to cause disease in a number of economically important plants worldwide. In the southeastern United States, PSV is widespread in forage legumes and is considered a major constraint to productivity and stand longevity (McLaughlin et al. 1992). It is one of the principal viruses associated with clover decline in the southeast (McLaughlin and Boykin 1988). In 2002, this virus (Fig. 1) was reported in the forage legume rhizoma or perennial peanut, Arachis glabrata Benth. (Blount et al. 2002). Perennial peanut was brought into Florida from Bra- zil in 1936. In general, the perennial peanut is well adapted to the light sandy soils of the southern Gulf Coast region of the U.S. It is drought-tolerant, grows well on low-fertility soils and is relatively free from disease or insect pest problems. The rela- tively impressive forage yields of some accessions makes the perennial peanut a promising warm-sea- son perennial forage legume for the southern Gulf Coast. Due to its high-quality forage, locally grown perennial peanut hay increasingly competes for the million plus dollar hay market currently satisfied by imported alfalfa (Medicago sativa L). There are ap- proximately 25,000 acres of perennial peanut in Ala- bama, Georgia and Florida combined. About 1000 acres are planted as living mulch in citrus groves. Fig. 1. A field of ‘Florigraze’ showing the yellowing symptoms of Peanut Popular forage cultivars include ‘Arbrook’ and Stunt Virus. -

Insect Classification Standards 2020
RECOMMENDED INSECT CLASSIFICATION FOR UGA ENTOMOLOGY CLASSES (2020) In an effort to standardize the hexapod classification systems being taught to our students by our faculty in multiple courses across three UGA campuses, I recommend that the Entomology Department adopts the basic system presented in the following textbook: Triplehorn, C.A. and N.F. Johnson. 2005. Borror and DeLong’s Introduction to the Study of Insects. 7th ed. Thomson Brooks/Cole, Belmont CA, 864 pp. This book was chosen for a variety of reasons. It is widely used in the U.S. as the textbook for Insect Taxonomy classes, including our class at UGA. It focuses on North American taxa. The authors were cautious, presenting changes only after they have been widely accepted by the taxonomic community. Below is an annotated summary of the T&J (2005) classification. Some of the more familiar taxa above the ordinal level are given in caps. Some of the more important and familiar suborders and families are indented and listed beneath each order. Note that this is neither an exhaustive nor representative list of suborders and families. It was provided simply to clarify which taxa are impacted by some of more important classification changes. Please consult T&J (2005) for information about taxa that are not listed below. Unfortunately, T&J (2005) is now badly outdated with respect to some significant classification changes. Therefore, in the classification standard provided below, some well corroborated and broadly accepted updates have been made to their classification scheme. Feel free to contact me if you have any questions about this classification. -

In Vitro Tissue Culture of Wild Arachis
IN VIRTO AND IN VIVO EVALUATION OF Arachis paraguariensis AND A. glabrata GERMPLASM By OLUBUNMI OLUFUNBI AINA A DISSERTATION PRESENTED TO THE GRADUATE SCHOOL OF THE UNIVERSITY OF FLORIDA IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF DOCTOR OF PHILOSOPHY UNIVERSITY OF FLORIDA 2011 1 © 2011 Olubunmi O. Aina 2 To my late father Isaac O. Ajani 3 ACKNOWLEDGMENTS It is a great privilege to be mentored and advised by such a knowledgeable and experienced professor like Dr. Kenneth H. Quesenberry. I sincerely thank him for the opportunity to join his team, as well as for his patience and support. His desire to see his student succeed in every aspect of life is worthy of acknowledgment and emulation. I would like to express my gratitude to all the members of my supervisory committee, Drs. Mike Kane, Barry Tillman, Maria Gallo, and Yoana Newman for giving me so much support, reassurance, and inspiration throughout this project. I am also thankful to Dr. Fredy Altpeter whose assistance always exceeded expectation. The staff at the University of Florida College of Medicine Electron Microscopy Core Facility is acknowledged for their assistance with the histological studies. I am thankful to my past and present lab members, as well as Judy Dampier, Gearry Durden, Justin McKinney and Jim Boyer for their help with the field evaluation aspect of this study. I am thankful to April, Bensa, and their entire family, as well as the families of other soccer moms who have invited my son to their home for sleepovers when I needed to work overnight on this dissertation. -

Great Lakes Entomologist the Grea T Lakes E N Omo L O G Is T Published by the Michigan Entomological Society Vol
The Great Lakes Entomologist THE GREA Published by the Michigan Entomological Society Vol. 45, Nos. 3 & 4 Fall/Winter 2012 Volume 45 Nos. 3 & 4 ISSN 0090-0222 T LAKES Table of Contents THE Scholar, Teacher, and Mentor: A Tribute to Dr. J. E. McPherson ..............................................i E N GREAT LAKES Dr. J. E. McPherson, Educator and Researcher Extraordinaire: Biographical Sketch and T List of Publications OMO Thomas J. Henry ..................................................................................................111 J.E. McPherson – A Career of Exemplary Service and Contributions to the Entomological ENTOMOLOGIST Society of America L O George G. Kennedy .............................................................................................124 G Mcphersonarcys, a New Genus for Pentatoma aequalis Say (Heteroptera: Pentatomidae) IS Donald B. Thomas ................................................................................................127 T The Stink Bugs (Hemiptera: Heteroptera: Pentatomidae) of Missouri Robert W. Sites, Kristin B. Simpson, and Diane L. Wood ............................................134 Tymbal Morphology and Co-occurrence of Spartina Sap-feeding Insects (Hemiptera: Auchenorrhyncha) Stephen W. Wilson ...............................................................................................164 Pentatomoidea (Hemiptera: Pentatomidae, Scutelleridae) Associated with the Dioecious Shrub Florida Rosemary, Ceratiola ericoides (Ericaceae) A. G. Wheeler, Jr. .................................................................................................183 -

Centomologica: -'F
:1 |II || ISSN 0001-561X AdTA| CENTOMOLOGICA: -'F. NNICA I A:_:1 $-** ; R. E. Linn vuori | Heteortera of Yemen and Siouth Yemenll 0 ,~~~~~~~~~~~~~~~~~~~I Vo41.4 1989 : ANNALES ENTOMOLOGICI FEMNNICI ACTA ENTOMOLOGICA FENNICA Published since 1935, four numbers a year. Published since 1947, monographs Annual subscription FIM 150, in Finland at irregular intervals. FIM 120. Price variable. Address: Zoological Museum, P. Rautatiek. 13, SF-00100 Helsinki, Finland. Publishers Suomen Hy6nteistieteellinen Seura Entomological Society of Finland - Societas Entomologica Fennica Entomologiska Foreningen i Helsingfors - Helsingin Hyonteistieteellinen Yhdistys Societas Entomologica Helsingforsiensis Editorial Board Chairman: A. Jansson (chief editor) Other members: K. Heliovaara (assistant editor of Acta), L. Hulddn (secretary, assistant editor), R. livarinen (treasurer), H. Krogerus, i. Mannerkoski, H. Silfverberg (editor of Acta) Board of Trustees President: E. Kangas Other members: 0. Bistrom, 1. Terds, A. Pekkarinen, R. Rosengren (vice president) Annales Entomologici Fennici publishes scientific papers, notes and reviews based principally on Finnish entomological investigations. Monographs and other longer articles are directed to Acta Entomologica Fennica, articles of mainly Nordic interest to Notulae Entomologicae. Contributors are requested to take into consideration the style and format of articles in recently published volumes. Two copies of each manuscript must be submitted with the original. As modern techniques often allow printing directly from computer diskettes, the editor should be informed if the manuscript is written on a word processor. The journals are cited selectively by Bibliographie der Pflanzenschutz-Literatur of Biologische Bundesanstaft for Land- und Forstwirtschaft, Biological Abstracts of the Biosciences Information Service, Current Contents (Series Agriculture, Biology & Environmental Sciences) of Institute for Scientific Information, Entomology Abstracts of Information Retrieval Limited, and Review of Applied Entomology (Series A. -

Template B V3.0 (Beta): Created by J. Nail 06/2015 Evaluation of Kudzu Bug As a Pest in Mississippi Soybean Production Systems
Template B v3.0 (beta): Created by J. Nail 06/2015 Evaluation of kudzu bug as a pest in Mississippi soybean production systems By TITLE PAGE William Michael McRight Jr. A Thesis Submitted to the Faculty of Mississippi State University in Partial Fulfillment of the Requirements for the Degree of Master of Science in Agricultural Life Sciences in the Department of Biochemistry, Molecular Biology, Entomology, and Plant Pathology Mississippi State, Mississippi May 2018 Copyright by COPYRIGHT PAGE William Michael McRight Jr. 2018 Evaluation of kudzu bug as a pest in Mississippi soybean production systems By APPROVAL PAGE William Michael McRight Jr. Approved: ____________________________________ Fred R. Musser (Director of Thesis) ____________________________________ Angus L. Catchot Jr. (Director of Thesis) ____________________________________ Donald R. Cook (Committee Member) ____________________________________ Jeffrey Gore (Committee Member) ____________________________________ Kenneth Willeford (Graduate Coordinator) ____________________________________ George M. Hopper Dean College of Agriculture and Life Sciences Name: William Michael McRight Jr. ABSTRACT Date of Degree: May 1, 2018 Institution: Mississippi State University Major Field: Agricultural Life Sciences Major Professors: Dr. Fred R. Musser and Dr. Angus L. Catchot Jr. Title of Study: Evaluation of kudzu bug as a pest in Mississippi soybean production systems Pages in Study: 60 Candidate for Degree of Master of Science The kudzu bug is an invasive species to the United States, and it has recently become a problem in the southern U.S. Experiments were conducted to examine the potential damage to vegetative stage soybean. Foliar insecticides and neonicotinoid seed treatments were studied to determine the efficacy of those treatments against kudzu bugs. Kudzu bug population densities of adults and nymphs along with egg masses were observed to determine peak activity in kudzu and soybean. -

Characterization of the Arachis (Leguminosae) D Genome Using Fluorescence in Situ Hybridization (FISH) Chromosome Markers and Total Genome DNA Hybridization
Genetics and Molecular Biology, 31, 3, 717-724 (2008) Copyright © 2008, Sociedade Brasileira de Genética. Printed in Brazil www.sbg.org.br Research Article Characterization of the Arachis (Leguminosae) D genome using fluorescence in situ hybridization (FISH) chromosome markers and total genome DNA hybridization Germán Robledo1 and Guillermo Seijo1,2 1Instituto de Botánica del Nordeste, Corrientes, Argentina. 2Facultad de Ciencias Exactas y Naturales y Agrimensura, Universidad Nacional del Nordeste, Corrientes, Argentina. Abstract Chromosome markers were developed for Arachis glandulifera using fluorescence in situ hybridization (FISH) of the 5S and 45S rRNA genes and heterochromatic 4’-6-diamidino-2-phenylindole (DAPI) positive bands. We used chro- mosome landmarks identified by these markers to construct the first Arachis species ideogram in which all the ho- mologous chromosomes were precisely identified. The comparison of this ideogram with those published for other Arachis species revealed very poor homeologies with all A and B genome taxa, supporting the special genome con- stitution (D genome) of A. glandulifera. Genomic affinities were further investigated by dot blot hybridization of biotinylated A. glandulifera total DNA to DNA from several Arachis species, the results indicating that the D genome is positioned between the A and B genomes. Key words: chromosome markers, DAPI bands, rDNA loci, dot blot hybridization, genome relationships. Received: June 29, 2007; Accepted: November 22, 2007. Introduction row et al., 2001; Simpson, 2001; Mallikarjuna, 2002; Mal- The genus Arachis comprises 80 wild species and the likarjuna et al., 2004). For this reason, great efforts have cultivated crop Arachis hypogaea L. (Fabales, Legumi- been directed towards understanding the relationships be- nosae) commonly known as groundnut or peanut. -

Evolution of the Insects
CY501-C08[261-330].qxd 2/15/05 11:10 PM Page 261 quark11 27B:CY501:Chapters:Chapter-08: 8 TheThe Paraneopteran Orders Paraneopteran The evolutionary history of the Paraneoptera – the bark lice, fold their wings rooflike at rest over the abdomen, but thrips true lice, thrips,Orders and hemipterans – is a history beautifully and Heteroptera fold them flat over the abdomen, which reflected in structure and function of their mouthparts. There probably relates to the structure of axillary sclerites and other is a general trend from the most generalized “picking” minute structures at the base of the wing (i.e., Yoshizawa and mouthparts of Psocoptera with standard insect mandibles, Saigusa, 2001). to the probing and puncturing mouthparts of thrips and Relationships among paraneopteran orders have been anopluran lice, and the distinctive piercing-sucking rostrum discussed by Seeger (1975, 1979), Kristensen (1975, 1991), or beak of the Hemiptera. Their mouthparts also reflect Hennig (1981), Wheeler et al. (2001), and most recently by diverse feeding habits (Figures 8.1, 8.2, Table 8.1). Basal Yoshizawa and Saigusa (2001). These studies generally agree paraneopterans – psocopterans and some basal thrips – are on the monophyly of the order Hemiptera and most of its microbial surface feeders. Thysanoptera and Hemiptera suborders and a close relationship of the true lice (order independently evolved a diet of plant fluids, but ancestral Phthiraptera) with the most basal group, the “bark lice” (Pso- heteropterans were, like basal living families, predatory coptera), which comprise the Psocodea. One major issue is insects that suction hemolymph and liquified tissues out of the position of thrips (order Thysanoptera), which either their prey. -

SCIENTIFIC NOTES on Coptosoma Variegatum HERRICH-SCHAEFFER, 1838 (HEMIPTERA: PLATASPIDAE): a POTENTIAL PEST of MANGO FLOWER in MALAYSIA
SCIENTIFIC NOTES ON Coptosoma variegatum HERRICH-SCHAEFFER, 1838 (HEMIPTERA: PLATASPIDAE): A POTENTIAL PEST OF MANGO FLOWER IN MALAYSIA Nurul Huda, A.1, 2*, Che Salmah, M.R.2, Hamdan, A.2 & Abdul Razak, M.N.3 1Department of Plant Science, Kulliyyah of Science, International Islamic University Malaysia, 25200 Kuantan, Pahang, Malaysia. 2School of Biological Sciences, Universiti Sains Malaysia, 11800 Penang, Malaysia. 3Faculty of Plantation and Agro-technology, Universiti Teknologi MARA, 02600 Arau, Perlis, Malaysia. *Corresponding author email: [email protected] ABSTRACT The incidence of infestation by a black stink bug Coptosoma variegatum (Hemiptera: Plataspidae) on mango Mangifera indica L. (Anacardiaceae) tree was scientifically reported for the first time in Malaysia. This insect is commonly known as home invader and legume pest. Abundance of this insect was monitored on mango panicles by 15 minutes hourly collection from 0800 h until 1500 h at 4-day interval from the beginning of flowering until all flowers dried up (12-28 February 2013 and 28 January 2014 – 7 March 2014). Five hundred twenty-six individuals collected during the study period with drastic increase observed in second season. However, their infestation on mango flowers was not fully evident therefore was suggested as a potential pest for mango flower in Malaysia solely due to their appearance on mango panicles. Nevertheless, co-occurrence of this sap-sucking insect with other secondary pests may pose a serious economic implication on the productivity of the crop. Thus, more research regarding this insect biology is required so that control requirement can be identified to maximize mango production in Malaysia. -

Jewel Bugs of Australia (Insecta, Heteroptera, Scutelleridae)1
© Biologiezentrum Linz/Austria; download unter www.biologiezentrum.at Jewel Bugs of Australia (Insecta, Heteroptera, Scutelleridae)1 G. CASSIS & L. VANAGS Abstract: The Australian genera of the Scutelleridae are redescribed, with a species exemplar of the ma- le genitalia of each genus illustrated. Scanning electron micrographs are also provided for key non-ge- nitalic characters. The Australian jewel bug fauna comprises 13 genera and 25 species. Heissiphara is described as a new genus, for a single species, H. minuta nov.sp., from Western Australia. Calliscyta is restored as a valid genus, and removed from synonymy with Choerocoris. All the Australian species of Scutelleridae are described, and an identification key is given. Two new species of Choerocoris are des- cribed from eastern Australia: C. grossi nov.sp. and C. lattini nov.sp. Lampromicra aerea (DISTANT) is res- tored as a valid species, and removed from synonymy with L. senator (FABRICIUS). Calliphara nobilis (LIN- NAEUS) is recorded from Australia for the first time. Calliphara billardierii (FABRICIUS) and C. praslinia praslinia BREDDIN are removed from the Australian biota. The identity of Sphaerocoris subnotatus WAL- KER is unknown and is incertae sedis. A description is also given for the Neotropical species, Agonoso- ma trilineatum (FABRICIUS); a biological control agent recently introduced into Australia to control the pasture weed Bellyache Bush (Jatropha gossypifolia, Euphorbiaceae). Coleotichus borealis DISTANT and C. (Epicoleotichus) schultzei TAUEBER are synonymised with C. excellens (WALKER). Callidea erythrina WAL- KER is synonymized with Lampromicra senator. Lectotype designations are given for the following taxa: Coleotichus testaceus WALKER, Coleotichus excellens, Sphaerocoris circuliferus (WALKER), Callidea aureocinc- ta WALKER, Callidea collaris WALKER and Callidea curtula WALKER.