Formation and Properties of the Whey Protein/-Casein Complexes In
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
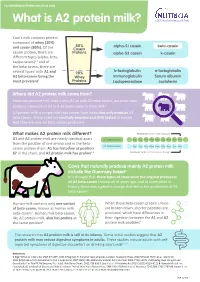
What Is A2 Protein Milk?
For Healthcare Professional use only What is A2 protein milk? Cow’s milk contains protein composed of whey (20%) 80% alpha-S1 casein beta-casein and casein (80%). Of the Casein casein protein, there are Proteins alpha-S2 casein k-casein different types (alpha, beta, kappa casein)1,2 and of the beta-casein, there are b-lactoglobulin a-lactoglobulin several types’ with A1 and 20% A2 beta-casein being the Whey Immunoglobulin Serum albumin most prevalent3 Proteins Lactoperoxidase Lactoferrin Where did A2 protein milk come from? Cows can produce milk that is only A1 or only A2 beta-casein, but most cows produce a mixture of A1 and A2 beta-casein in their milk4 A2 protein milk is simply milk that comes from cows that only produce A2 beta-casein. These cows are carefully selected and DNA tested to ensure that they are only A2 beta-casein producers What makes A2 protein milk different? Position 67(proline hinders cleavage) A1 and A2 protein milk are nearly identical apart A2 beta-casein Val Tyr Pro Phe Pro Gly Pro Iie Pro from the position of one amino acid in the beta- A1 beta-casein casein protein chain: A1 has histadine at position Val Tyr Pro Phe Pro Gly Pro Iie His 67 in the chain, and A2 protein milk has proline4,5 Position 67(histidine readily allows cleavage) Cows that naturally produce mainly A2 protein milk include the Guernsey breed4 It is thought that these types of cows were the original producers of A2 beta-casein thousands of years ago, and at some point in history there was a genetic change that led to the production of A1 beta-casein6 Human milk contains only one variant When these beta-casein protein chains of beta-casein, known as human milk are broken down, shorter peptides are beta-casein7. -

Suggested Protein Supplements
Suggested Protein Supplements Choose supplements that provide 100-200 calories, 20-30 grams of protein, and less than 5 grams of sugar per standard serving. A good supplement will provide at least 15 grams of protein per 100 calories. Supplement Calories Protein Sugar Protein Other Where to Purchase (serving size) (grams) (grams) Source Ready to Drink (RTD) Elevation 160 30 1 Milk GF Aldi, online High Performance Kosher Protein Shake (11 fl oz) Ensure Max 150 30 1 Milk GF/LF CVS, Rite Aid, Shopper’s, Target, (11 fl oz) Kosher Walgreen’s, Walmart, Weis, online Equate 160 30 1 Milk GF Walmart, online High Performance Kosher (11 fl oz) Fairlife 150 30 2 Milk GF/LF BJ’s, Sam’s Club, online Nutrition Plan Kosher (11.5 fl oz) GNC Lean Shake 25 170 25 2 Milk LF GNC, online (14 fl oz) Orgain Organic Protein 150 26 1 Milk GF Costco, Rite Aid, Safeway, Nutritional Kosher Target, Vitamin Shoppe, (14 fl oz) Walgreen’s, Whole Foods, online Orgain Organic Protein 150 21 0 Pea GF/LF Costco, Rite Aid, Safeway, Vegan Kosher Target, Vitamin Shoppe, (14 fl oz) Walgreen’s, Whole Foods, online Premier Protein 160 30 1 Milk GF BJ’s, Costco, CVS, Sam’s Club, (11 fl oz) Kosher Food Lion, Giant, Harris Teeter, Rite Aid, Safeway, Target, Walgreen’s, Walmart, 7 Eleven, online Pure Protein Milk GF Costco, Sam’s Club, BJ’s, Giant, Shake (11 fl oz can) 150-170 35 1 Safeway, Vitamin Shoppe, Complete Shake (11 fl oz) 140 30 <1 Walmart, online Quest 160 30 1 Milk GF CVS, Giant, Target, Vitamin (11 fl oz) Kosher Shoppe, Walmart Unjury 110 20 2 Milk Kosher Unjury.com, -

Casein Proteins As a Vehicle to Deliver Vitamin D3
Casein Proteins as a Vehicle to Deliver Vitamin D3: Fortification of Dairy Products with vitamin D3 and Bioavailability of Vitamin D3 from Fortified Mozzarella Cheese Baked with Pizza by Banaz Al-khalidi A thesis submitted in conformity with the requirements for the degree of Master of Science Graduate Department of Nutritional Sciences University of Toronto © Copyright by Banaz Al-khalidi (2012) Casein proteins as a vehicle to deliver vitamin D3: Fortification of dairy products with vitamin D3 and Bioavailability of vitamin D3 from fortified Mozzarella cheese baked with pizza Banaz Al-khalidi Master of Science Department of Nutritional Sciences, Faculty of Medicine University of Toronto 2012 ABSTRACT Current vitamin D intakes in Canada are inadequate. The extension of vitamin D fortification to additional foods may be an effective and appropriate strategy for increasing vitamin D intakes in the general population. Cheese is potentially an ideal candidate for vitamin D fortification. We introduce the potential use of casein proteins as a vehicle for vitamin D3 fortification in industrially made cheeses where we found that over 90% of vitamin D3 added to milk was retained in both Cheddar and Mozzarella cheeses. Use of casein proteins for vitamin D3 fortification did not fully prevent vitamin D3 loss into whey. However the loss was minimized to approximately 8%. We then show that vitamin D3 is bioavailable from fortified Mozzarella cheese baked with pizza suggesting that the high temperature baking process does not significantly breakdown vitamin D3. Our findings could have important implications in increasing fortified food options for Canadians. ii TABLE OF CONTENTS ABSTRACT .................................................................................................................................. -

Milk Allergy Vs. Lactose Intolerance
Milk Allergy vs. Lactose Intolerance KNOW THE DIFFERENCE COW’S MILK ALLERGY LacTOSE INTOLERANCE Cause Cause • Your immune system treats the proteins • Your body cannot break down the sugar in milk as a threat. in milk (lactose), which causes symptoms • This causes your immune system to in your digestive system. attack the protein and your body makes • Does not involve the immune system. a reaction. Age Age • Usually starts before age 1 • Usually starts after age 2 • Most children outgrow it by age 3 Possible Symptoms Possible Symptoms Skin Only affects digestion • Red, itchy, rash, swelling • Diarrhea Airways • Stomach cramps • Trouble breathing or swallowing, runny • Nausea nose, coughing • Vomiting • Tingling, itchy, swollen lips or mouth • Bloating Digestion • Gas • Diarrhea, stomach cramps, nausea, vomiting Anaphylaxis • Severe allergic reaction, can result in death if not treated How to Diagnose How to Diagnose • Diagnosis should always be done by a • Diagnosis should always be done by a qualified medical professional, such as qualified medical professional, such as an allergy specialist. Talk to your health an allergy specialist. Talk to your health care provider about the possible tests care provider about the possible tests that can be done. that can be done. Treatment Treatment • Do not consume any food or drinks that • Reduce intake of food and drinks that contain milk protein. contain lactose (many people with • Breastfeed, if possible, exclusively for lactose intolerance can consume some 6 months to age 2 and beyond. lactose with no symptoms). • If formula feeding, talk to your health • If you have symptoms, choose foods care provider about a formula that and drinks that are low lactose or would be appropriate for your infant. -

Gluten Free Casein Free Diet
GLUTEN FREE, CASEIN FREE DIET The gluten free, casein free (GFCF) diet has been shown to be helpful for individuals with allergies to these particular foods and specifically in the management of autistic spectrum disorder (ASD). Proteins found in grain and dairy products, known as gluten and casein respectively, are believed to be poorly broken down in the digestive tracts in some people. When these proteins are not digested properly they can be absorbed intact into blood circulation. These proteins can affect the brain by crossing the blood-brain barrier and binding to opioid receptors. This can affect mood, concentration, mental performance and pain tolerance (i.e. in autistic children this will increase their pain threshold). Research has shown significant improvement in several conditions, including schizophrenia and autism, following a GFCF diet. In a survey of over 3500 parents of autistic children, it was reported that 70% found a GFCF diet improved behavior, eye contact and socialisation, concentration and learning. It is recommended to follow the GFCF diet strictly for at least 6 months to assess the benefit of this diet. Below is a list of foods containing gluten and casein that are suggested to avoid, plus a list of alternative GFCF choices. RECOMMENDED AVOID GRAINS AND • Amaranth • Baked Beans unless gluten free LEGUMES • Basmati Rice • Flours: Wheat flour, wholemeal flour, • Beans bakers flour, semolina, barley, rye • Brown Rice (avoid battered or crumbed food) • Buckwheat • Wheat including durum, semolina, • Chickpea triticale, -

A2 Milk Popularity on the Rise by BEN VERSTEEG, SEMEX SALES & PRODUCT SPECIALIST
A2 Milk Popularity on the Rise BY BEN VERSTEEG, SEMEX SALES & PRODUCT SPECIALIST A hot topic in the dairy industry today is the Beta-casein protein production is controlled by the growing popularity of A2 beta-casein milk among combination of any two of these variants (ie. A1A2) as consumers and dairy farmers. Farmers in many all cows carry two alleles. These alleles are co-dominant, regions of the world are being incentivised to meaning that cows that carry two different variants produce A2 milk to meet the growing demand in (heterozygous) will produce equal amounts of each what is considered to be a healthier alternative protein that they carry, while cows that carry two copies to conventional dairy (Zoetis, 2015). However the of the same allele (homozygous) will produce only science behind this trend remains controversial that protein (Woodford, 2007). This makes achieving and is not well understood by many consumers a homozygous A2 herd exclusively through genetic and producers. The goal of this article is to selection a possibility for dairy producers. While a present an assessment of the facts as they are quick conversion to A2 would be possible via genetic currently known and explain Semex’s A2 brand. testing and selective culling of A1 carriers, a more sound approach could be a step-wise approach of genetic Milk is composed of several solid components including selection for A2A2 sires in advance of conversion to minerals, lactose, fat and protein. There are three notable mitigate the need for A1 culling. casein milk proteins: alpha, kappa, and beta-casein - the protein of interest to us in this article (Zoetis, 2015). -

Hidden Dairy “Cheat Sheet” Business Card-Sized (Cut out and Fold in the Middle)
From kellymom.com… Hidden Dairy “Cheat Sheet” Business card-sized (cut out and fold in the middle) Personal use only. If you would like to distribute this handout to clients or patients, please visit www.kellymom.com/bookstore/handouts for more information. Dairy Ingredients and Hidden Dairy Ingredients that MAY contain milk protein: Artificial butter flavor, Butter, Butter fat, Buttermilk, Chocolate, Flavorings (natural or artificial), High protein Butter oil, Casein, Caseinates (ammonia, calcium, flour, Hot Dogs, Luncheon Meat, Margarine, Simplesse, magnesium, potassium, sodium), Cheese, Cottage Sausage, Starter Distillate. cheese, Cream, Curds, Custard, Ghee, Goat’s milk, Avoid "deli" meats, because the slicers frequently are used Half & half, Hydrolysates (casein, milk protein, protein, to cut both meat and cheese products. Also, some deli whey, whey protein), Kefir, Koumiss, Lactalbumin, meats contain dairy products. Lactalbumin phosphate, Lactoglobulin, Lactose, Lactulose, Milk (condensed, derivative, powder, dry, Kosher labeling: A product label marked Parve or evaporated, low fat, malted, non fat, protein, skim, solids, Pareve is certified dairy-free. A product with a circled whole), Milkfat, Nougat, Paneer, Pudding, Rennet “U” on the label (with NO other symbols or letters) is casein, Sour cream, Sour cream solids, Sour milk solids, Parve. A "D" or "DE" on a product label next to a circled Whey (in any form including delactosed, demineralized, "K" or circled "U" may indicate the presence of milk protein concentrate, sweet), Yogurt protein. — kellymom.com . -

Milk Whey Protein Standard
Milk Whey Protein Standard Product Definition Milk Whey Protein is obtained from bovine milk or skim milk by the removal of casein and non-protein constituents from milk so that the finished dry product contains not less than 25% protein. It is obtained by microfiltration and/or chromatography of milk or skim milk and may be preceded or followed by ultrafiltration, nanofiltration, evaporation, dialysis, or any other safe and suitable process in which all or part of the lactose, minerals and moisture may be removed. Products cannot be produced through any process or combination of processes that include enzymatic coagulation of protein and/or acid precipitation of protein in bovine milk or skim milk. Milk Whey Protein products with a protein content less than 89.5% protein are referred to as Milk Whey Protein Concentrates (or mWPC). Milk Whey Protein products with a protein content ≥89.5% protein on a dry matter basis are referred to as Milk Whey Protein Isolates (or mWPI). Composition Several different mWPC or mWPI products are commercially available. These may include: Product Protein % Fat % Lactose % Ash % Moisture % mWPC 34 Min. 33.5% Max. 2.0% Max. 55.0% Max. 7.50% Max. 6.0% mWPC 80 Min. 79.5%* Max. 2.0% Max. 13.0% Max. 5.0% Max. 6.0% mWPI 90 Min. 89.5%* Max. 1.5% Max. 4.0% Max. 4.5% Max. 6.0% (*) Protein content ≥ 79.5% is reported on a dry basis, all other parameters are reported “as is” Microbiological Standards and Methods of Analysis Parameter Standard Test Method Standard Plate Count 30,000cfu/g max AOAC 966.23 Coliform Bacteria 10cfu/g max AOAC 989.10 (Petrifilm Salmonella Neg. -

Product Recovery and BOD Reduction Davisco Foods International, Inc. Stephen Raab Advisor: Matt Domski On-Site Supervisor: Jeff Shodean Who I Am
Product Recovery and BOD Reduction Davisco Foods International, Inc. Stephen Raab Advisor: Matt Domski On-site Supervisor: Jeff Shodean Who I Am • University of Notre Dame undergraduate • Class of 2016 • B.S. in Chemical Engineering • Lives in Bloomington, MN • Longtime fan of the U of M Davisco-Le Sueur Overview • Le Sueur Cheese Company • Cheese • Lactose • Whey Protein • Food Ingredient Plant www.daviscofoods.com/locations/index.htm Company Mindset DOE Energy Star Program/Energy Savings Initiative -25% -25% -25% Energy Water GHGs Over 5 Years Incentives to Change • Annual water use: 180,500,000 gallons • $1.25 million annual BOD charges • Risk of exceeding pollution allowances • Floor Cheese • 184 pounds daily • “Costs twice” Process Overview Packaging Tower Curd Hopper Bag Pre-Press Pre-Fill Final Fill Ram Conveyor Barrel Cart Undergrade Tote Filtration/Water Polishing • Whey filtered of lactose, protein • Leftover water sent to polisher, purified via membrane filtration www.wheyoflife.org • COW water used for rinses Drying/Scrubber • Product dried through contact with hot air • Scrubber spray removes dry particles from air to ensure clean venting https://www.youtube.com/watch?v=uEizgsW-He4 Packaging Solutions Packaging Solutions, cont. Packaging Solutions, cont. 3.25” Economic Analysis-Packaging Implementation Annual Savings Recommendation Annual Savings ($) Payback Period (years) Status Cost (product) Catch Pans $1,000 7,100 lbs $3,650 0.27 Recommended Totes $50 270 lbs $183 0.27 Approved Automation Changes - 1547 lbs $2,939 Immediate -

The Structure and Function of Large Biological Molecules 5
The Structure and Function of Large Biological Molecules 5 Figure 5.1 Why is the structure of a protein important for its function? KEY CONCEPTS The Molecules of Life Given the rich complexity of life on Earth, it might surprise you that the most 5.1 Macromolecules are polymers, built from monomers important large molecules found in all living things—from bacteria to elephants— can be sorted into just four main classes: carbohydrates, lipids, proteins, and nucleic 5.2 Carbohydrates serve as fuel acids. On the molecular scale, members of three of these classes—carbohydrates, and building material proteins, and nucleic acids—are huge and are therefore called macromolecules. 5.3 Lipids are a diverse group of For example, a protein may consist of thousands of atoms that form a molecular hydrophobic molecules colossus with a mass well over 100,000 daltons. Considering the size and complexity 5.4 Proteins include a diversity of of macromolecules, it is noteworthy that biochemists have determined the detailed structures, resulting in a wide structure of so many of them. The image in Figure 5.1 is a molecular model of a range of functions protein called alcohol dehydrogenase, which breaks down alcohol in the body. 5.5 Nucleic acids store, transmit, The architecture of a large biological molecule plays an essential role in its and help express hereditary function. Like water and simple organic molecules, large biological molecules information exhibit unique emergent properties arising from the orderly arrangement of their 5.6 Genomics and proteomics have atoms. In this chapter, we’ll first consider how macromolecules are built. -

Casein Products
CASEIN PRODUCTS Casein is the principal protein found in cow’s milk from which it has been extracted commercially for most of the 20th century. It is responsible for the white, opaque appearance of milk in which it is combined with calcium and phosphorus as clusters of casein molecules, called “micelles”. The major uses of casein until the 1960s were in technical, non-food applications such as adhesives for wood, in paper coating, leather finishing and in synthetic fibres, as well as plastics for buttons, buckles etc. During the past 30 years, however, the principal use of casein products has been as an ingredient in foods to enhance their physical (so-called “functional”) properties, such as whipping and foaming, water binding and thickening, emulsification and texture, and to improve their nutrition. In New Zealand, casein is precipitated from the skim milk that is produced after centrifugal separation of whole milk. The skim milk may be acidified to produce acid casein or treated with an enzyme, resulting in the so-called rennet casein. The precipitated casein curd is separated from the whey, washed and dried. Water-soluble derivatives of acid caseins, produced by reaction with alkalis, are called caseinates. INTRODUCTION The amount of casein in cow’s whole milk varies according to the breed of cow and stage of lactation, but is generally in the range 24-29 g L-1. Casein contains 0.7-0.9% phosphorus, covalently bound to the protein by a serine ester linkage. Casein is consequently known as a phospho-protein. All the amino acids that are essential to man are present in casein in high proportions, with the possible exception of cysteine. -

Dairy Ingredients and Hidden Dairy
kellymom.com Hidden Dairy “Cheat Sheet” Dairy Ingredients and Hidden Dairy Ingredients that MAY contain milk protein: Artificial butter flavor, Butter, Butter fat, Buttermilk, Chocolate, Flavorings (natural or artificial), High protein Butter oil, Casein, Caseinates (ammonia, calcium, flour, Hot Dogs, Luncheon Meat, Margarine, Simplesse, magnesium, potassium, sodium), Cheese, Cottage Sausage, Starter Distillate. cheese, Cream, Curds, Custard, Ghee, Goat’s milk, Avoid "deli" meats, because the slicers frequently are used Half & half, Hydrolysates (casein, milk protein, protein, to cut both meat and cheese products. Also, some deli whey, whey protein), Kefir, Koumiss, Lactalbumin, meats contain dairy products. Lactalbumin phosphate, Lactoglobulin, Lactose, Lactulose, Milk (condensed, derivative, powder, dry, Kosher labeling: A product label marked Parve or evaporated, low fat, malted, non fat, protein, skim, solids, Pareve is certified dairy-free. A product with a circled whole), Milkfat, Nougat, Paneer, Pudding, Rennet “U” on the label (with NO other symbols or letters) is casein, Sour cream, Sour cream solids, Sour milk solids, Parve. A "D" or "DE" on a product label next to a circled Whey (in any form including delactosed, demineralized, "K" or circled "U" may indicate the presence of milk protein concentrate, sweet), Yogurt protein. — kellymom.com Dairy Ingredients and Hidden Dairy Ingredients that MAY contain milk protein: Artificial butter flavor, Butter, Butter fat, Buttermilk, Chocolate, Flavorings (natural or artificial), High protein Butter oil, Casein, Caseinates (ammonia, calcium, flour, Hot Dogs, Luncheon Meat, Margarine, Simplesse, magnesium, potassium, sodium), Cheese, Cottage Sausage, Starter Distillate. cheese, Cream, Curds, Custard, Ghee, Goat’s milk, Avoid "deli" meats, because the slicers frequently are used Half & half, Hydrolysates (casein, milk protein, protein, to cut both meat and cheese products.