The Macroecology of Population Dynamics: Taxonomic and Biogeographic Patterns in Population Cycles
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Population, Consumption & the Environment
12/11/2009 Population, Consumption & the Environment Alex de Sherbinin Center for International Earth Science Information Network (CIESIN), the Earth Institute at Columbia University Population-Environment Research Network 2 1 12/11/2009 Why is this important? • Global GDP is 20 times higher today than it was in 1900, having grown at a rate of 2.7% per annum (population grew at the rate of 13%1.3% p.a.) • CO2 emissions have grown at an annual rate of 3.5% since 1900, reaching 100 million metric tons of carbon in 2001 • The ecological footprint, a composite measure of consumption measured in hectares of biologically productive land, grew from 4.5 to 14.1 billion hectares between 1961 and 2003, and it is now 25% more than Earth’s “biocapacity ” • For CO2 emissions and footprints, the per capita impacts of high‐income countries are currently 6 to 10 times higher than those in low‐income countries 3 Outline 1. What kind of consumption is bad for the environment? 2. How are population dynamics and consumption linked? 3. Who is responsible for environmentally damaging consumption? 4. What contributions can demographers make to the understanding of consumption? 5. Conclusion: The challenge of “sustainable consumption” 4 2 12/11/2009 What kind of consumption is bad for the environment? SECTION 2 5 What kind of consumption is bad? “[Consumption is] human transformations of materials and energy. [It] is environmentally important to the extent that it makes materials or energy less available for future use, and … through its effects on biophysical systems, threatens hhlthlfththill”human health, welfare, or other things people value.” - Stern, 1997 • Early focus on “wasteful consumption”, conspicuous consumption, etc. -

Chapter 15 Biogeography and Dispersal
Chapter 15 Biogeography and dispersal Rob Hengeveld and Lia Hemerik Introduction This chapter evaluates the role of dispersal in biogeographical processes and their re- sulting patterns. We consider dispersal as a local process, which comprises the com- bined movements of individual organisms, but which can dominate processes even at the scale of continents. If this is correct, it is no longer possible to separate local ecological processes from those at broad, geographical scales. However, biogeo- graphical processes differ from those happening in one or a few localities; at the broader scales,there are additional processes occurring which are only evident when examined from this wider perspective. We integrate biogeography with ecology, explaining broad-scale effects, ranging from processes happening locally as the result of responses of individual organisms to perpetual changes in living conditions in heterogeneous space. The models to be used cannot be those traditional in population dynamics with a dispersal parameter plugged in, but must be spatially explicit. Only a broad-scale perspective of con- tinual redistribution of large groups of individuals or reproductive propagules can give dispersal its biological and biogeographical significance. Our general thesis in this chapter is that adaptation in non-uniform space enables individuals to cope effectively with environmental variation in time. In our analyses of spatially adaptive processes, we concentrate on principles rather than on details of specific phenomena, such as types of distance distribution. We therefore formulate these principles in terms of simple Poisson processes.In spe- cific cases, these distributions can be replaced by more complex ones which may fit better. -

Can More K-Selected Species Be Better Invaders?
Diversity and Distributions, (Diversity Distrib.) (2007) 13, 535–543 Blackwell Publishing Ltd BIODIVERSITY Can more K-selected species be better RESEARCH invaders? A case study of fruit flies in La Réunion Pierre-François Duyck1*, Patrice David2 and Serge Quilici1 1UMR 53 Ӷ Peuplements Végétaux et ABSTRACT Bio-agresseurs en Milieu Tropical ӷ CIRAD Invasive species are often said to be r-selected. However, invaders must sometimes Pôle de Protection des Plantes (3P), 7 chemin de l’IRAT, 97410 St Pierre, La Réunion, France, compete with related resident species. In this case invaders should present combina- 2UMR 5175, CNRS Centre d’Ecologie tions of life-history traits that give them higher competitive ability than residents, Fonctionnelle et Evolutive (CEFE), 1919 route de even at the expense of lower colonization ability. We test this prediction by compar- Mende, 34293 Montpellier Cedex, France ing life-history traits among four fruit fly species, one endemic and three successive invaders, in La Réunion Island. Recent invaders tend to produce fewer, but larger, juveniles, delay the onset but increase the duration of reproduction, survive longer, and senesce more slowly than earlier ones. These traits are associated with higher ranks in a competitive hierarchy established in a previous study. However, the endemic species, now nearly extinct in the island, is inferior to the other three with respect to both competition and colonization traits, violating the trade-off assumption. Our results overall suggest that the key traits for invasion in this system were those that *Correspondence: Pierre-François Duyck, favoured competition rather than colonization. CIRAD 3P, 7, chemin de l’IRAT, 97410, Keywords St Pierre, La Réunion Island, France. -

The Basics of Population Dynamics Greg Yarrow, Professor of Wildlife Ecology, Extension Wildlife Specialist
The Basics of Population Dynamics Greg Yarrow, Professor of Wildlife Ecology, Extension Wildlife Specialist Fact Sheet 29 Forestry and Natural Resources Revised May 2009 All forms of wildlife, regardless of the species, will respond to changes in density dependence. These concepts are important for landowners habitat, hunting or trapping, and weather conditions with fluctuations and natural resource managers to understand when making decisions in animal numbers. Most landowners have probably experienced affecting wildlife on private land. changes in wildlife abundance from year to year without really knowing why there are fewer individuals in some years than others. How Many Offspring Can Wildlife Have? In many cases, changes in abundance are normal and to be expected. Most people realize that some wildlife species can produce more The purpose of the information presented here is to help landowners offspring than others. Bobwhite quail are genetically programmed to lay understand why animal numbers may vary or change. While a number an average of 14 eggs per clutch. Each species has a maximum genetic of important concepts will be discussed, one underlying theme should reproductive potential or biotic potential. always be remembered. Regardless of whether property is managed or not in any given year, there is always some change in the habitat, Biotic potential describes a population’s ability to grow over time however small. Wildlife must adjust to this change and, therefore, no through reproduction. Most bat species are likely to produce one population is ever the same from one year to the next. offspring per year. In contrast, a female cottontail rabbit will have a litter size of approximately 5. -

An Empirical Analysis in Kenya, Senegal, and Eswatini
sustainability Article Energy–Climate–Economy–Population Nexus: An Empirical Analysis in Kenya, Senegal, and Eswatini Samuel Asumadu Sarkodie 1,* , Emmanuel Ackom 2 , Festus Victor Bekun 3,4 and Phebe Asantewaa Owusu 1 1 Nord University Business School, Post Box 1490, 8049 Bodo, Norway; [email protected] 2 Department of Technology, Management and Economics, UNEP DTU Partnership, UN City Campus, Denmark Technical University (DTU), Marmorvej 51, 2100 Copenhagen, Denmark; [email protected] 3 Faculty of Economics Administrative and Social sciences, Istanbul Gelisim University, 34310 Istanbul, Turkey; [email protected] 4 Department of Accounting, Analysis, and Audit, School of Economics and Management, South Ural State University, 76, Lenin Aven., 454080 Chelyabinsk, Russia * Correspondence: [email protected] Received: 29 June 2020; Accepted: 29 July 2020; Published: 31 July 2020 Abstract: Motivated by the Sustainable Development Goals (SDGs) and its impact by 2030, this study examines the relationship between energy consumption (SDG 7), climate (SDG 13), economic growth and population in Kenya, Senegal and Eswatini. We employ a Kernel Regularized Least Squares (KRLS) machine learning technique and econometric methods such as Dynamic Ordinary Least Squares (DOLS), Fully Modified Ordinary Least Squares (FMOLS) regression, the Mean-Group (MG) and Pooled Mean-Group (PMG) estimation models. The econometric techniques confirm the Environmental Kuznets Curve (EKC) hypothesis between income level and CO2 emissions while the machine learning method confirms the scale effect hypothesis. We find that while CO2 emissions, population and income level spur energy demand and utilization, economic development is driven by energy use and population dynamics. This demonstrates that income, population growth, energy and CO2 emissions are inseparable, but require a collective participative decision in the achievement of the SDGs. -

Chapter 11 Population Dynamics
APES Chapter 4 Human Population Factors in human population size A. Population Growth =(Births + immigration) - (deaths + emigration) B. When factors are stable ZPG = Zero population Growth C. Use Crude birth and death rate - # per 1000 people D. Rate of change % = birth rate – death rate x 100 1,000 people E. World has slowed population rate but still growing very fast F. Fertility rates 1. Replacement level fertility – couple has 2.1 children 2. Total Fertility rate – TFR – estimate of number of children a woman would have under current age specific birth rates a. better measure b. different in different parts of the world - developed 1.6 - developing 3.4 in developing G. Fertility rates in US – more of a problem because of Americans high resource use. 1. drop in TFR but population still growing – Why? - Large number of baby boomers still in childbearing years - Increase in number of teen mothers US has highest rate of any industrialized countries UN studies say US teens not more sexually active just less likely to know how to prevent pregnancies or less willing to use them. 77% of all teen mothers go on welfare within 5 years - Higher fertility rates non Caucasian mothers - High levels of legal and illegal immigrants – accounts for more than 40% of growth H. Factors that affect Birth and fertility rates 1. level of education and affluence 2. Importance of children in the work force 3. Urbanization 4. Cost of raising and educating children 5. Educational and Employment opportunities for women 6. Infant mortality rates 7. Average age of marriage 8. -

Dynamics of Ecological Communities in Variable Environments –Local and Spatial Processes
Linköping Studies in Science and Technology Dissertation No. 1431 Dynamics of ecological communities in variable environments –local and spatial processes Linda Kaneryd Department of Physics, Chemistry and Biology Theoretical Biology Linköping University SE-581 83 Linköping, Sweden Linköping, Mars 2012 Linköping Studies in Science and Technology, Dissertation No. 1431 Kaneryd, L. Dynamics of ecological communities in variable environments – local and spatial processes Copyright © Linda Kaneryd, unless otherwise noted Also available from Linköping University Electronic Press http://www.ep.liu.se/ ISBN: 978 – 91 – 7519 – 946 – 7 ISSN: 0345 – 7524 Front cover: Designed by Johan Sjögren, photo by courtesy of Dan Tommila Printed by LiU-Tryck, Linköping 2012 Abstract The ecosystems of the world are currently facing a variety of anthropogenic perturbations, such as climate change, fragmentation and destruction of habitat, overexploitation of natural resources and invasions of alien species. How the ecosystems will be affected is not only dependent on the direct effects of the perturbations on individual species but also on the trophic structure and interaction patterns of the ecological community. Of particular current concern is the response of ecological communities to climate change. Increased global temperature is expected to cause an increased intensity and frequency of weather extremes. A more unpredictable and more variable environment will have important consequences not only for individual species but also for the dynamics of the entire community. If we are to fully understand the joint effects of a changing climate and habitat fragmentation, there is also a need to understand the spatial aspects of community dynamics. In the present work we use dynamic models to theoretically explore the importance of local (Paper I and II) and spatial processes (Paper III-V) for the response of multi-trophic communities to different kinds of perturbations. -

Modeling Population Dynamics
Modeling Population Dynamics Andr´eM. de Roos Modeling Population Dynamics Andr´eM. de Roos Institute for Biodiversity and Ecosystem Dynamics University of Amsterdam Science Park 904, 1098 XH Amsterdam, The Netherlands E-mail: [email protected] December 4, 2019 Contents I Preface and Introduction 1 1 Introduction 3 1.1 Some modeling philosophy . .3 II Unstructured Population Models in Continuous Time 5 2 Modelling population dynamics 7 2.1 Describing a population and its environment . .7 2.1.1 The population or p-state . .7 2.1.2 The individual or i-state . .8 2.1.3 The environmental or E-condition . .9 2.2 Population balance equation . 10 2.3 Characterizing the population . 11 2.4 Population-level and per capita rates . 12 2.5 Model building . 15 2.5.1 Exponential population growth . 15 2.5.2 Logistic population growth . 16 2.5.3 Two-sexes population growth . 17 2.6 Parameters and state variables . 18 2.7 Deterministic and stochastic models . 19 3 Single ordinary differential equations 21 3.1 Explicit solutions . 21 3.2 Numerical integration . 23 3.3 Analyzing flow patterns . 24 3.4 Steady states and their stability . 28 3.5 Units and non-dimensionalization . 32 3.6 Existence and uniqueness of solutions . 35 3.7 Epilogue . 36 4 Competing for resources 37 4.1 Intraspecific competition . 38 i ii CONTENTS 4.1.1 Growth of yeast in a closed container . 38 4.1.2 Bacterial growth in a chemostat . 40 4.1.3 Asymptotic dynamics . 43 4.1.4 Phase-plane methods and graphical analysis . -

State of World Population 2009 Population of World State
state state of world population 2009 state of world population 2009 Facing a changing world: women, population and climate Facing a changing Facing world: women, population and climate United Nations Population Fund 220 East 42nd Street New York, NY 10017 USA www.unfpa.org USD $17.50 ISBN 978-0-89714-958-7 sales no. E.09.III.H.1 E/27,000/2009 Seal the Deal! is a United Nations-led campaign to promote a fair, balanced and effective agreement on climate change when governments meet in Printed on recycled paper. Copenhagen in December 2009. Editorial team The State of World Population 2009 Lead Author and Researcher: Robert Engelman, Worldwatch Institute The United Nations Environment Programme contributed the foundation Chapter 1 contributors: Janet Macharia, Kaveh Zahedi and Bubu Jallow of for Chapter 1, “Elements of climate change.” The International Organization the United Nations Environment Programme for Migration and the Office of the United Nations High Commissioner for Chapter 3 contributors: Philippe Boncour of the International Organization Refugees jointly drafted Chapter 3, “On the move.” for Migration and José Riera of the Office of the United Nations High Commissioner for Refugees The editorial team also thanks the Inter Press News Agency and the Editor: Richard Kollodge Integrated Regional Information Networks for permission to reprint stories Editorial Associate: Triana D’Orazio from their news services. Editorial and Administrative Associate: Mirey Chaljub The indicators in the report were generously contributed by the Population Acknowledgements Division of the United Nations Department of Economic and Social Affairs, UNESCO Institute for Statistics, the World Health Organization, the Food The editorial team is especially grateful to UNFPA’s Technical Division for and Agriculture Organization of the United Nations, the World Bank, the contributing to the development of the report and reviewing drafts: Pamela UNFPA/NIDI Resource Flows Project and the Population Reference Bureau. -
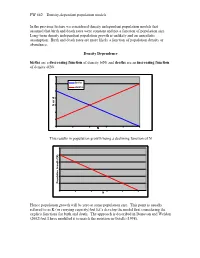
FW 662 – Density-Dependent Population Models in the Previous
FW 662 – Density-dependent population models In the previous lecture we considered density independent population models that assumed that birth and death rates were constant and not a function of population size. Long-term density independent population growth is unlikely and an unrealistic assumption. Birth and death rates are more likely a function of population density or abundance. Density Dependence births are a decreasing function of density b(N) and deaths are an increasing function of density d(N). births deaths d b or N This results in population growth being a declining function of N ) N ( th (f w o r G on ti a l pu o P N Hence population growth will be zero at some population size. This point is usually referred to as K (or carrying capacity) but let’s develop the model first considering the explicit functions for birth and death. The approach is described in Donovan and Weldon (2002) but I have modified it to match the notation in Gotelli (1998). FW 662 – Density-dependent population models We need two new terms to account for changes in per capita birth and death rates a= the amount by which the per capita birth rate changes in response to an addition of one individual to the population. c= ditto for death rate… Our density independent discrete model was a difference equation expressed as: N t+1 = N t + bN t − dN t We replace b and d (the density independent birth rate and death rate) with: b − aN t and d + cN t Now our new density dependent model looks like N t+1 = N t + ()b − aN t N t − (d + cN t )N t Population growth rate is not easy to visualize from this equation. -

Was Malthus Right? Economic Growth and Population Dynamics∗
Was Malthus Right? Economic Growth and Population Dynamics∗ Jesús Fernández-Villaverde University of Pennsylvania December 3, 2001 Abstract This paper studies the relationship between population dynamics and economic growth. Prior to the Industrial Revolution increases in total output were roughly matched by increases in population. In contrast, during the last 150 years, incre- ments in per capita income have coexisted with slow population growth. Why are income and population growth no longer positively correlated? This paper presents a new answer, based on the role of capital-specific technological change,thatprovides a unifying account of lower population growth and sustained economic growth. An overlapping generations model with capital-skill complementarity and endogenous fertility, mortality and education is constructed and parametrized to match Eng- lish data. The key finding is that the observed fall in the relative price of capital accounts for more than 60% of the fall in fertility and over 50% of the increase in in- come per capita in England occurred during the demographic transition. Additional experiments show that neutral technological change or the reduction in mortality cannot account for the fall in fertility. ∗Department of Economics, 160 McNeil Building, 3718 Locust Walk Philadelphia, PA 19104-6297. E- mail: [email protected]. Thanks to seminars participants at several institutions, Hal Cole, Juan Carlos Conesa, Karsten Jeske, Narayana Kocherlakota, Dirk Krueger, Edward Prescott and especially Lee Ohanian for useful comments. 1 1. Introduction This paper studies the relationship between population dynamics and economic growth. It makes two contributions. First, it presents a new propagation channel from technological change into population growth: the combination of capital-specific technological change and capital-skill complementarity. -
6. Distribution Patterns & Community Ecology
6. Distribution Patterns & Community Ecology Population Dynamics Community Dynamics Community Succession Zonation Faunal driven Environmentally driven Global Biogeography Dr Laura J. Grange ([email protected]) 20th April 2010 Reading: Levinton, Chapter 17 “Biotic Diversity in the Ocean” and Smith et al. 2008 Levels Individual An organism physiologically independent from other individuals Population A group of individuals of the same species that are responding to the same environmental variables Community A group of populations of different species all living in the same place Ecosystem A group of inter-dependent communities in a single geographic area capable of living nearly independently of other ecosystems Biosphere All living things on Earth and the environment with which they interact Population Dynamics What do populations need to survive? Suitable environment Populations limited Temps O2 Pressure Etc. Range of Tolerance Population Dynamics Environment selects traits that “work” r strategists K strategists What makes a healthy population? Minimum Viable Population (MVP) “Population size necessary to ensure 90-95% probability of survival 100-1000 years in the future” i.e. Enough reproducing males and females to keep the population going Community Dynamics Communities A group of populations of different species all living in the same place Each population has a “role” in the community Primary Producers Turn chemical energy into food energy Photosynthesizers, Chemosynthesizers Consumers Trophic