Anthrax As a Biological Weapon, 2002 Updated Recommendations for Management
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Developing Drugs for Prophylaxis of Inhalational Anthrax Guidance for Industry
Anthrax: Developing Drugs for Prophylaxis of Inhalational Anthrax Guidance for Industry U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER) May 2018 Clinical/Antimicrobial Anthrax: Developing Drugs for Prophylaxis of Inhalational Anthrax Guidance for Industry Additional copies are available from: Office of Communications, Division of Drug Information Center for Drug Evaluation and Research Food and Drug Administration 10001 New Hampshire Ave., Hillandale Bldg., 4th Floor Silver Spring, MD 20993-0002 Phone: 855-543-3784 or 301-796-3400; Fax: 301-431-6353; Email: [email protected] https://www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/default.htm U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER) May 2018 Clinical/Antimicrobial TABLE OF CONTENTS I. INTRODUCTION............................................................................................................. 1 II. BACKGROUND ............................................................................................................... 2 A. Historical Background................................................................................................................... 2 B. Indication for Prophylaxis of Inhalational Anthrax ................................................................... 2 III. DEVELOPMENT PROGRAM ....................................................................................... 3 A. General -

Amerithrax Investigative Summary
The United States Department of Justice AMERITHRAX INVESTIGATIVE SUMMARY Released Pursuant to the Freedom of Information Act Friday, February 19, 2010 TABLE OF CONTENTS I. THE ANTHRAX LETTER ATTACKS . .1 II. EXECUTIVE SUMMARY . 4 A. Overview of the Amerithrax Investigation . .4 B. The Elimination of Dr. Steven J. Hatfill as a Suspect . .6 C. Summary of the Investigation of Dr. Bruce E. Ivins . 6 D. Summary of Evidence from the Investigation Implicating Dr. Ivins . .8 III. THE AMERITHRAX INVESTIGATION . 11 A. Introduction . .11 B. The Investigation Prior to the Scientific Conclusions in 2007 . 12 1. Early investigation of the letters and envelopes . .12 2. Preliminary scientific testing of the Bacillus anthracis spore powder . .13 3. Early scientific findings and conclusions . .14 4. Continuing investigative efforts . 16 5. Assessing individual suspects . .17 6. Dr. Steven J. Hatfill . .19 7. Simultaneous investigative initiatives . .21 C. The Genetic Analysis . .23 IV. THE EVIDENCE AGAINST DR. BRUCE E. IVINS . 25 A. Introduction . .25 B. Background of Dr. Ivins . .25 C. Opportunity, Access and Ability . 26 1. The creation of RMR-1029 – Dr. Ivins’s flask . .26 2. RMR-1029 is the source of the murder weapon . 28 3. Dr. Ivins’s suspicious lab hours just before each mailing . .29 4. Others with access to RMR-1029 have been ruled out . .33 5. Dr. Ivins’s considerable skill and familiarity with the necessary equipment . 36 D. Motive . .38 1. Dr. Ivins’s life’s work appeared destined for failure, absent an unexpected event . .39 2. Dr. Ivins was being subjected to increasing public criticism for his work . -

2001 Anthrax Letters
Chapter Five: 2001 Anthrax Letters Author’s Note: The analysis and comments regarding the communication efforts described in this case study are solely those of the authors; this analysis does not represent the official position of the FDA. This case was selected because it is one of the few major federal efforts to distribute medical countermeasures in response to an acute biological incident. These events occurred more than a decade ago and represent the early stages of US biosecurity preparedness and response; however, this incident serves as an excellent illustration of the types of communication challenges expected in these scenarios. Due in part to the extended time since these events and the limited accessibility of individual communications and messages, this case study does not provide a comprehensive assessment of all communication efforts. In contrast to the previous case studies in this casebook, the FDA’s role in the 2001 anthrax response was relatively small, and as such, this analysis focuses principally on the communication efforts of the CDC and state and local public health agencies. The 2001 anthrax attacks have been studied extensively, and the myriad of internal and external assessments led to numerous changes to response and communications policies and protocols. The authors intend to use this case study as a means of highlighting communication challenges strictly within the context of this incident, not to evaluate the success or merit of changes made as a result of these events. Abstract The dissemination of Bacillus anthracis via the US Postal Service (USPS) in 2001 represented a new public health threat, the first intentional exposure to anthrax in the United States. -

NECROPHILIC and NECROPHAGIC SERIAL KILLERS Approval Page
Running head: NECROPHILIC AND NECROPHAGIC SERIAL KILLERS Approval Page: Florida Gulf Coast University Thesis APPROVAL SHEET This thesis is submitted in partial fulfillment of the requirements for the degree of Master of Science Christina Molinari Approved: August 2005 Dr. David Thomas Committee Chair / Advisor Dr. Shawn Keller Committee Member The final copy of this thesis has been examined by the signatories, and we find that both the content and the form meet acceptable presentation standards of scholarly work in the above mentioned discipline. NECROPHILIC AND NECROPHAGIC SERIAL KILLERS 1 Necrophilic and Necrophagic Serial Killers: Understanding Their Motivations through Case Study Analysis Christina Molinari Florida Gulf Coast University NECROPHILIC AND NECROPHAGIC SERIAL KILLERS 2 Table of Contents Abstract ........................................................................................................................................... 5 Literature Review............................................................................................................................ 7 Serial Killing ............................................................................................................................... 7 Characteristics of sexual serial killers ..................................................................................... 8 Paraphilia ................................................................................................................................... 12 Cultural and Historical Perspectives -

Molecular Subtyping of Bacillus Anthracis and the 2001 Bioterrorism-Associated Anthrax Outbreak, United States Alex R
BIOTERRORISM-RELATED ANTHRAX Molecular Subtyping of Bacillus anthracis and the 2001 Bioterrorism-Associated Anthrax Outbreak, United States Alex R. Hoffmaster,* Collette C. Fitzgerald,* Efrain Ribot,* Leonard W. Mayer,* and Tanja Popovic* Molecular subtyping of Bacillus anthracis played an important role in differentiating and identifying strains during the 2001 bioterrorism-associated outbreak. Because B. anthracis has a low level of genetic variabil- ity, only a few subtyping methods, with varying reliability, exist. We initially used multiple-locus variable- number tandem repeat analysis (MLVA) to subtype 135 B. anthracis isolates associated with the outbreak. All isolates were determined to be of genotype 62, the same as the Ames strain used in laboratories. We sequenced the protective antigen gene (pagA) from 42 representative outbreak isolates and determined they all had a pagA sequence indistinguishable from the Ames strain (PA genotype I). MLVA and pagA sequencing were also used on DNA from clinical specimens, making subtyping B. anthracis possible with- out an isolate. Use of high-resolution molecular subtyping determined that all outbreak isolates were indis- tinguishable by the methods used and probably originated from a single source. In addition, subtyping rapidly identified laboratory contaminants and nonoutbreak–related isolates. he recent bioterrorism-associated anthrax outbreak dem- subtype 26 diverse B. anthracis isolates into six PA genotypes T onstrated the need for rapid molecular subtyping of Bacil- (8). Although sequencing of pagA results in limited numbers lus anthracis isolates. Numerous methods, including multiple- of subtypes, it does have the added benefit of determining if locus enzyme electrophoresis (MEE) and multiple-locus the pagA gene has been altered or engineered. -

Lessons from the Anthrax Attacks Implications for US
Lessons from the Anthrax Attacks Implications for US. Bioterrorism Preparedness A Report on a National Forum on Biodefense Author David Heymart Research Assistants Srusha Ac h terb erg, L Joelle Laszld Organized by the Center for Strategic and International Studies and the Defense Threat Reduction Agency --CFr”V? --.....a DlSTRf BUTION This report ISfor official use only; distribution authorized to U S. government agencies, designated contractors, and those with an official need Contains information that may be exempt from public release under the Freedom of Information Act. exemption number 2 (5 USC 552); exemption number 3 (’lo USC 130).Approvalof the Defense Threat Reduction Agency prior to public release is requrred Contract Number OTRAM-02-C-0013 For Official Use Only About CSIS For four decades, the Center for Stravgic and Internahonal Studies (CSIS)has been dedicated 10 providing world leaders with strategic insights on-and pohcy solubons tcurrentand emergtng global lssues CSIS IS led by John J Hamre, former L S deputy secretary of defense It is guided by a board of trustees chaired by former U S senator Sam Nunn and consistlng of prominent individuals horn both the public and private sectors The CSIS staff of 190 researchers and support staff focus pnrnardy on three subjecr areas First, CSIS addresses the fuU spectrum of new challenges to national and mternabonal security Second, it maintains resident experts on all of the world's myor geographical regions Third, it IS committed to helping to develop new methods of governance for the global age, to this end, CSIS has programs on technology and pubhc policy, International trade and finance, and energy Headquartered in Washington, D-C ,CSIS IS pnvate, bipartlsan, and tax-exempt CSIS docs not take specific policy positions, accordygly, all views expressed herein should be understood to be solely those ofthe author Spousor. -
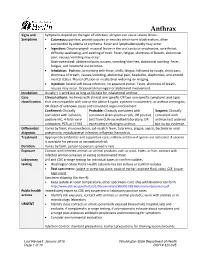
Anthrax Reporting and Investigation Guideline
Anthrax Signs and Symptoms depend on the type of infection; all types can cause severe illness: Symptoms • Cutaneous: painless, pruritic papules or vesicles which form black eschars, often surrounded by edema or erythema. Fever and lymphadenopathy may occur. • Ingestion: Oropharyngeal: mucosal lesion in the oral cavity or oropharynx, sore throat, difficulty swallowing, and swelling of neck. Fever, fatigue, shortness of breath, abdominal pain, nausea/vomiting may occur. Gastrointestinal: abdominal pain, nausea, vomiting/diarrhea, abdominal swelling. Fever, fatigue, and headache are common. • Inhalation: Biphasic, presenting with fever, chills, fatigue, followed by cough, chest pain, shortness of breath, nausea/vomiting, abdominal pain, headache, diaphoresis, and altered mental status. Pleural effusion or mediastinal widening on imaging. • Injection: Severe soft tissue infection; no apparent eschar. Fever, shortness of breath, nausea may occur. Occasional meningeal or abdominal involvement. Incubation Usually < 1 week but as long as 60 days for inhalational anthrax Case Clinical criteria: An illness with at least one specific OR two non-specific symptoms and signs classification that are compatible with one of the above 4 types, systemic involvement, or anthrax meningitis; OR death of unknown cause and consistent organ involvement Confirmed: Clinically Probable: Clinically consistent with Suspect: Clinically consistent with isolation, consistent Gram-positive rods, OR positive consistent with positive IHC, 4-fold rise in test from CLIA-accredited laboratory, OR anthrax test ordered antibodies, PCR, or LF MS epi evidence relating to anthrax but no epi evidence Differential Varies by form; mononucleosis, cat-scratch fever, tularemia, plague, sepsis, bacterial or viral diagnosis pneumonia, mycobacterial infection, influenza, hantavirus Treatment Appropriate antibiotics and supportive care; anthrax antitoxin if spores are activated. -

Bioterrorism & Biodefense
Hugh-Jones et al. J Bioterr Biodef 2011, S3 Bioterrorism & Biodefense http://dx.doi.org/10.4172/2157-2526.S3-001 Review Article Open Access The 2001 Attack Anthrax: Key Observations Martin E Hugh-Jones1*, Barbara Hatch Rosenberg2 and Stuart Jacobsen3 1Professor Emeritus, Louisiana State University; Anthrax Moderator, ProMED-mail, USA 2Sloan-Kettering Institute for Cancer Research and State Univ. of NY-Purchase (retired); Scientists Working Group on CBW, Center for Arms Control and Non-Proliferation, USA 3Technical Consultant Silicon Materials, Dallas, TX,USA Abstract Unresolved scientificquestions, remaining ten years after the anthrax attacks, three years after the FBI accused a dead man of perpetrating the 2001 anthrax attacks singlehandedly, and more than a year since they closed the case without further investigation, indictment or trial, are perpetuating serious concerns that the FBI may have accused the wrong person of carrying out the anthrax attacks. The FBI has not produced concrete evidence on key questions: • Where and how were the anthrax spores in the attack letters prepared? There is no material evidence of where the attack anthrax was made, and no direct evidence that any specific individual made the anthrax, or mailed it. On the basis of a number` of assumptions, the FBI has not scrutinized the most likely laboratories. • How and why did the spore powders acquire the high levels of silicon and tin found in them? The FBI has repeatedly insisted that the powders in the letters contained no additives, but they also claim that they have not been able to reproduce the high silicon content in the powders, and there has been little public mention of the extraordinary presence of tin. -

Necro, Murder Ya Life
Necro, Murder Ya Life Stabbin your face With a butcher knife thats really long I'll make you feel this song When I beat you down to it I don't care if I appear on it I'll do it I represent the death rap, get your head cracked open So we stair at your brain I don't care if you think I'm insane cause I take respect this serious So if you dis-re-spect, your an idiot Stick an ice pick in your neck till you bleed like a period Damb you kid, fam you kid And if you dont, then you will when I fuck fear in you bitch Put it through you violently, silently Walk up to you, you have no idea its me Rockin a mask, poppin you fast with a glock, with a silencer When you die you say; It's Necro, the sicko, let go, I'm a jack it you fagget, let that flow Die like a man, if you can, but you cant, so you won't cause your a male hoe Yo, I run this shit Put guns through your chest Shootin breast milk all over your cereal Run your shit, your clothes, your shoes And if you refuse-get your ass killed all over material Brutal, sadistic, the only way to rip shit, I'm a stay cryptic Till the end of time The only day you'll be doper then me with a rhyme is when I quit, dip shit It 'il never go down like that I'll still be around, from the ground I'll rap As a corpse, as a verse thats driving the tell all demons on earth how to survive an "L" My death rap is attacking you Your gettin stabbed in the brain with a verbal knife You better watch your step, and show some respect Or else I have to murder your life My death rap is attacking you Your gettin stabbed in the brain with a verbal knife You better watch your step, and show some respect Or else I have to murder your life Chopping you up you fagget And droppi Necro - Murder Ya Life w Teksciory.pl. -

Vaccination of Rhesus Macaques with the Anthrax Vaccine Adsorbed
CLINICAL AND VACCINE IMMUNOLOGY, Nov. 2010, p. 1753–1762 Vol. 17, No. 11 1556-6811/10/$12.00 doi:10.1128/CVI.00174-10 Copyright © 2010, American Society for Microbiology. All Rights Reserved. Vaccination of Rhesus Macaques with the Anthrax Vaccine Adsorbed Vaccine Produces a Serum Antibody Response That Effectively Neutralizes Receptor-Bound Protective Antigen In Vitroᰔ Kristin H. Clement,1* Thomas L. Rudge, Jr.,1 Heather J. Mayfield,1 Lena A. Carlton,1 Arelis Hester,1 Nancy A. Niemuth,1 Carol L. Sabourin,1 April M. Brys,1 and Conrad P. Quinn2 Battelle Memorial Institute, 505 King Avenue, Columbus, Ohio 43201,1 and Meningitis and Vaccine Preventable Diseases Branch, Division of Bacterial Diseases, National Center for Immunization and Respiratory Diseases, Centers for Disease Control and Prevention, Atlanta, Georgia 303332 Received 29 April 2010/Returned for modification 17 June 2010/Accepted 19 August 2010 Anthrax toxin (ATx) is composed of the binary exotoxins lethal toxin (LTx) and edema toxin (ETx). They have separate effector proteins (edema factor and lethal factor) but have the same binding protein, protective antigen (PA). PA is the primary immunogen in the current licensed vaccine anthrax vaccine adsorbed (AVA [BioThrax]). AVA confers protective immunity by stimulating production of ATx-neutralizing antibodies, which could block the intoxication process at several steps (binding of PA to the target cell surface, furin cleavage, toxin complex formation, and binding/translocation of ATx into the cell). To evaluate ATx neutral- ization by anti-AVA antibodies, we developed two low-temperature LTx neutralization activity (TNA) assays that distinguish antibody blocking before and after binding of PA to target cells (noncomplexed [NC] and receptor-bound [RB] TNA assays). -

Gao-15-80, Anthrax
United States Government Accountability Office Report to Congressional Requesters December 2014 ANTHRAX Agency Approaches to Validation and Statistical Analyses Could Be Improved GAO-15-80 December 2014 ANTHRAX Agency Approaches to Validation and Statistical Analyses Could Be Improved Highlights of GAO-15-80, a report to congressional requesters Why GAO Did This Study What GAO Found In 2001, the FBI investigated an After the 2001 Anthrax attacks, the genetic tests that were conducted by the intentional release of B. anthracis, a Federal Bureau of Investigation’s (FBI) four contractors were generally bacterium that causes anthrax, which scientifically verified and validated, and met the FBI’s criteria. However, GAO was identified as the Ames strain. found that the FBI lacked a comprehensive approach—or framework—that could Subsequently, FBI contractors have ensured standardization of the testing process. As a result, each of the developed and validated several contractors developed their tests differently, and one contractor did not conduct genetic tests to analyze B. anthracis verification testing, a key step in determining whether a test will meet a user’s samples for the presence of certain requirements, such as for sensitivity or accuracy. Also, GAO found that the genetic mutations. The FBI had contractors did not develop the level of statistical confidence for interpreting the previously collected and maintained testing results for the validation tests they performed. Responses to future these samples in a repository. incidents could be improved by using a standardized framework for achieving GAO was asked to review the FBI’s minimum performance standards during verification and validation, and by genetic test development process and incorporating statistical analyses when interpreting validation testing results. -

The Role of Music in the Lives of Homeless Young People
Tuned Souls: The Role of Music in the Lives of Homeless Young People Jill P. Woelfer A dissertation submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy University of Washington 2014 Reading Committee: David Hendry, Chair Susan Kemp Batya Friedman Julie A. Hersberger Program Authorized to Offer Degree: The Information School ©Copyright 2014 Jill P. Woelfer University of Washington Abstract Tuned Souls: The Role of Music is the Lives of Homeless Young People Jill P. Woelfer Chair of the Supervisory Committee: Associate Professor, David G. Hendry The Information School Although music is considered to be an important part of adolescence and young adulthood, little is known about music and homeless youth. Accordingly, this dissertation research investigated the role of music in the lives of homeless young people, aged 15-25. The study was conducted in Seattle, Washington and Vancouver, British Columbia and engaged homeless young people (n=202) and service providers staff who work at agencies that provide support for homeless young people (n=24). Homeless young people completed surveys (n=202), design activities, which included drawing and story writing (n=149), and semi-structured interviews (n=40). Service providers completed semi-structured interviews (n=24). Data analysis included descriptive analysis of survey data and qualitative coding of the design activities and interview responses. Findings indicated that music was an important part of everyday life for homeless young people, who listened to music daily (98%), owned music players (89%), and had wide- ranging and eclectic tastes in music which did not vary based on location.