Feeding Practices for Captive Greater Kudus (Tragelaphus Strepsiceros) in UK Collections As Compared to Diets of Free-Ranging Specimens
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Population, Distribution and Conservation Status of Sitatunga (Tragelaphus Spekei) (Sclater) in Selected Wetlands in Uganda
POPULATION, DISTRIBUTION AND CONSERVATION STATUS OF SITATUNGA (TRAGELAPHUS SPEKEI) (SCLATER) IN SELECTED WETLANDS IN UGANDA Biological -Life history Biological -Ecologicl… Protection -Regulation of… 5 Biological -Dispersal Protection -Effectiveness… 4 Biological -Human tolerance Protection -proportion… 3 Status -National Distribtuion Incentive - habitat… 2 Status -National Abundance Incentive - species… 1 Status -National… Incentive - Effect of harvest 0 Status -National… Monitoring - confidence in… Status -National Major… Monitoring - methods used… Harvest Management -… Control -Confidence in… Harvest Management -… Control - Open access… Harvest Management -… Control of Harvest-in… Harvest Management -Aim… Control of Harvest-in… Harvest Management -… Control of Harvest-in… Tragelaphus spekii (sitatunga) NonSubmitted Detrimental to Findings (NDF) Research and Monitoring Unit Uganda Wildlife Authority (UWA) Plot 7 Kira Road Kamwokya, P.O. Box 3530 Kampala Uganda Email/Web - [email protected]/ www.ugandawildlife.org Prepared By Dr. Edward Andama (PhD) Lead consultant Busitema University, P. O. Box 236, Tororo Uganda Telephone: 0772464279 or 0704281806 E-mail: [email protected] [email protected], [email protected] Final Report i January 2019 Contents ACRONYMS, ABBREVIATIONS, AND GLOSSARY .......................................................... vii EXECUTIVE SUMMARY ....................................................................................................... viii 1.1Background ........................................................................................................................... -

Colors & Patterns in Nature
California State Standards K-LS1-1 Lesson Plan: Grade Level: Kindergarten Colors & Patterns in Nature Background All animals have specific colors and patterns that help them survive in their environment. Fish use the color of their scales to become invisible to flying predators by reflecting the sun. Walking sticks mimic the twigs of trees to hide. Even predators like the cheetah have special patterns to help them survive. Goal In this lesson students will observe the animals of Safari West and explore how their patterns and colors can provide clues to what type of habitat they live in and how it helps them survive. We will also examine the patterns that predators use to make themselves invisible to their prey. Before Your Visit Activity 1: For a fun class project before visiting Safari West, have students make binoculars. This requires two toilet paper rolls, glue, yarn, and pens/crayons. Explain what binoculars are used for and how they help scientists get a closer look at animals. Note to educators: The binoculars help young students focus on individual animals or specific morphological features (as described in the Background above). Activity 2: You may also want to have students make their own notebooks with four pages of b lank white paper so they can draw the various patterns, stripes or spots they see on some of the animals while on safari. Materials to Ask your students bring the ‘”binoculars” and notebooks they made in Bring the classroom. If they did not make their own notebook, you may want to provide them with pre-made versions. -

For Review Only 440 IUCN SSC Antelope Specialist Group
Manuscripts submitted to Journal of Mammalogy A Pliocene hybridisation event reconciles incongruent mitochondrial and nuclear gene trees among spiral-horned antelopes Journal:For Journal Review of Mammalogy Only Manuscript ID Draft Manuscript Type: Feature Article Date Submitted by the Author: n/a Complete List of Authors: Rakotoarivelo, Andrinajoro; University of Venda, Department of Zoology O'Donoghue, Paul; Specialist Wildlife Services, Specialist Wildlife Services Bruford, Michael; Cardiff University, Cardiff School of Biosciences Moodley, Yoshan; University of Venda, Department of Zoology adaptive radiation, interspecific hybridisation, paleoclimate, Sub-Saharan Keywords: Africa, Tragelaphus https://mc.manuscriptcentral.com/jmamm Page 1 of 34 Manuscripts submitted to Journal of Mammalogy 1 Yoshan Moodley, Department of Zoology, University of Venda, 2 [email protected] 3 Interspecific hybridization in Tragelaphus 4 A Pliocene hybridisation event reconciles incongruent mitochondrial and nuclear gene 5 trees among spiral-horned antelopes 6 ANDRINAJORO R. RAKATOARIVELO, PAUL O’DONOGHUE, MICHAEL W. BRUFORD, AND * 7 YOSHAN MOODLEY 8 Department of Zoology, University of Venda, University Road, Thohoyandou 0950, Republic 9 of South Africa (ARR, ForYM) Review Only 10 Specialist Wildlife Services, 102 Bowen Court, St Asaph, LL17 0JE, United Kingdom (PO) 11 Cardiff School of Biosciences, Sir Martin Evans Building, Cardiff University, Museum 12 Avenue, Cardiff, CF10 3AX, United Kingdom (MWB) 13 Natiora Ahy Madagasikara, Lot IIU57K Bis, Ampahibe, Antananarivo 101, Madagascar 14 (ARR) 15 16 1 https://mc.manuscriptcentral.com/jmamm Manuscripts submitted to Journal of Mammalogy Page 2 of 34 17 ABSTRACT 18 The spiral-horned antelopes (Genus Tragelaphus) are among the most phenotypically diverse 19 of all large mammals, and evolved in Africa during an adaptive radiation that began in the late 20 Miocene, around 6 million years ago. -

Animals of Africa
Silver 49 Bronze 26 Gold 59 Copper 17 Animals of Africa _______________________________________________Diamond 80 PYGMY ANTELOPES Klipspringer Common oribi Haggard oribi Gold 59 Bronze 26 Silver 49 Copper 17 Bronze 26 Silver 49 Gold 61 Copper 17 Diamond 80 Diamond 80 Steenbok 1 234 5 _______________________________________________ _______________________________________________ Cape grysbok BIG CATS LECHWE, KOB, PUKU Sharpe grysbok African lion 1 2 2 2 Common lechwe Livingstone suni African leopard***** Kafue Flats lechwe East African suni African cheetah***** _______________________________________________ Red lechwe Royal antelope SMALL CATS & AFRICAN CIVET Black lechwe Bates pygmy antelope Serval Nile lechwe 1 1 2 2 4 _______________________________________________ Caracal 2 White-eared kob DIK-DIKS African wild cat Uganda kob Salt dik-dik African golden cat CentralAfrican kob Harar dik-dik 1 2 2 African civet _______________________________________________ Western kob (Buffon) Guenther dik-dik HYENAS Puku Kirk dik-dik Spotted hyena 1 1 1 _______________________________________________ Damara dik-dik REEDBUCKS & RHEBOK Brown hyena Phillips dik-dik Common reedbuck _______________________________________________ _______________________________________________African striped hyena Eastern bohor reedbuck BUSH DUIKERS THICK-SKINNED GAME Abyssinian bohor reedbuck Southern bush duiker _______________________________________________African elephant 1 1 1 Sudan bohor reedbuck Angolan bush duiker (closed) 1 122 2 Black rhinoceros** *** Nigerian -

Seasonal Sightings
Avg. 26°C 3 days/month, 40 mm Avg. 25°C 9 days/month, 31 mm Forest Elephant, Forest Bualo, Bushbuck, Putty-nose Monkey, Guereza Colobus, Western Lowland Forest Elephant, Forest Bualo, Bushbuck, Putty-nose Monkey, Guereza Colobus, Western Lowland Gorilla, Palm Nut Gorilla, Palm Nut Vulture, Grey Parrot, Green Pigeon, Great Blue Turaco, Spotted Hyena, Sitatunga, B E R J A N Vulture, Grey Parrot, Green Pigeon, Great Blue Turaco, Spotted Hyena, Sitatunga, Marsh Mongoose, Demido’s Marsh Mongoose, Demido’s Galago, Grey Cheeked Mangabey, Moustached Monkey, De Brazza’s E M U A E C R Y Galago, Grey Cheeked Mangabey, Moustached Monkey, Giant Forest Hog, Red River Hog, Bongo, Palm Civet, Monkey, Giant Forest Hog, Red River Hog, Bongo, Palm Civet, Crowned Monkey, D Crowned Monkey, Congo Clawless Otter, Agile Mangabey, De Brazza’s Monkey, Pel’s Fishing Owl Congo Clawless Otter, Agile Mangabey, Pel’s Fishing Owl R F E E B B M R Avg. 26°C 5 days/month, 91 mm E U Avg. 28°C 8 days/month, 32 mm V A O R Y Forest Elephant, Forest Bualo, Bushbuck, Putty-nose Monkey, Guereza Colobus, Western N Forest Elephant, Forest Bualo, Bushbuck, Putty-nose Monkey, Guereza Colobus, Western Lowland Gorilla, Palm Nut Vulture, Grey Parrot, Green Pigeon, Great Blue Turaco, Spotted Lowland Gorilla, Palm Nut Vulture, Grey Parrot, Green Pigeon, Great Blue Turaco, Spotted Hyena, Sitatunga, Marsh Mongoose, Demido’s Galago, Grey Cheeked Mangabey, Hyena, Sitatunga, Marsh Mongoose, Demido’s Galago, Grey Cheeked Mangabey, Moustached Monkey, De Brazza’s Monkey, Giant Forest Hog, Red River Hog, Bongo, Palm Moustached Monkey, Giant Forest Hog, Red River Hog, Bongo, Palm Civet, Crowned Civet, Crowned Monkey, Congo Clawless Otter, Agile Mangabey, Pel’s Fishing Owl Monkey, Congo Clawless Otter, Agile Mangabey, De Brazza’s Monkey, Pel’s Fishing Owl R M E A B R Avg. -

Common Eland
Tragelaphus oryx – Common Eland recognised, though their validity has been in dispute (Thouless 2013): Tragelaphus o. livingstonii (Sclater 1864; Livingstone's Eland): also called kaufmanni, niediecki, selousi and triangularis. It is found in the Central Zambezian Miombo woodlands i.e. south- central Africa (Angola, Zambia, Democratic Republic of the Congo, Zimbabwe, Mozambique and Malawi). Livingstone's Eland has a brown pelt with up to twelve stripes. Tragelaphus o. oryx (Pallas 1766; Cape Eland): also called alces, barbatus, canna and oreas. This subspecies is found south of the Zambezi river (South Africa, Botswana and Namibia). The fur is tawny, and adults lose their stripes. Regional Red List status (2016) Least Concern Tragelaphus o. pattersonianus (Lydekker 1906; East National Red List status (2004) Least Concern African Eland or Patterson's Eland): also called Reasons for change No change billingae. It is found in east Africa extending into the Somali arid areas, hence its common name. Its coat Global Red List status (2008) Least concern can have up to 12 stripes. TOPS listing (NEMBA) None Tragelaphus o. oryx occurs throughout the larger part of South Africa, but the far northern Limpopo Province CITES listing None bordering Zimbabwe is regarded as a transitional zone Endemic No between T. o. oryx and T. o. livingstonii or an area where they overlap. This argues the case that they should rather During drought conditions Eland roam extensively be described as ecotypes (in ecotypes, it is common for in order to meet forage and water requirements; in continuous, gradual geographic variation to impose the southern Kalahari during abnormally dry analogous phenotypic and/or genetic variation; this conditions, Eland were found to cover more than situation is called cline.). -

Mammal Species Richness at a Catena and Nearby Waterholes During a Drought, Kruger National Park, South Africa
diversity Article Mammal Species Richness at a Catena and Nearby Waterholes during a Drought, Kruger National Park, South Africa Beanélri B. Janecke Animal, Wildlife & Grassland Sciences, University of the Free State, 205 Nelson Mandela Road, Park West, Bloemfontein 9301, South Africa; [email protected]; Tel.: +27-51-401-9030 Abstract: Catenas are undulating hillslopes on a granite geology characterised by different soil types that create an environmental gradient from crest to bottom. The main aim was to determine mammal species (>mongoose) present on one catenal slope and its waterholes and group them by feeding guild and body size. Species richness was highest at waterholes (21 species), followed by midslope (19) and sodic patch (16) on the catena. Small differences observed in species presence between zones and waterholes and between survey periods were not significant (p = 0.5267 and p = 0.9139). In total, 33 species were observed with camera traps: 18 herbivore species, 10 carnivores, two insectivores and three omnivores. Eight small mammal species, two dwarf antelopes, 11 medium, six large and six mega-sized mammals were observed. Some species might not have been recorded because of drought, seasonal movement or because they travelled outside the view of cameras. Mammal presence is determined by food availability and accessibility, space, competition, distance to water, habitat preferences, predators, body size, social behaviour, bound to territories, etc. The variety in body size and feeding guilds possibly indicates a functioning catenal ecosystem. This knowledge can be beneficial in monitoring and conservation of species in the park. Keywords: catena ecosystem; ephemeral mud wallows; habitat use; mammal variety; Skukuza area; Citation: Janecke, B.B. -

Jan 2021 ZSL Stocklist.Pdf (699.26
Zoological Society of London - January 2021 stocklist ZSL LONDON ZOO Status at 01.01.2021 m f unk Invertebrata Aurelia aurita * Moon jellyfish 0 0 150 Pachyclavularia violacea * Purple star coral 0 0 1 Tubipora musica * Organ-pipe coral 0 0 2 Pinnigorgia sp. * Sea fan 0 0 20 Sarcophyton sp. * Leathery soft coral 0 0 5 Sinularia sp. * Leathery soft coral 0 0 18 Sinularia dura * Cabbage leather coral 0 0 4 Sinularia polydactyla * Many-fingered leather coral 0 0 3 Xenia sp. * Yellow star coral 0 0 1 Heliopora coerulea * Blue coral 0 0 12 Entacmaea quadricolor Bladdertipped anemone 0 0 1 Epicystis sp. * Speckled anemone 0 0 1 Phymanthus crucifer * Red beaded anemone 0 0 11 Heteractis sp. * Elegant armed anemone 0 0 1 Stichodactyla tapetum Mini carpet anemone 0 0 1 Discosoma sp. * Umbrella false coral 0 0 21 Rhodactis sp. * Mushroom coral 0 0 8 Ricordea sp. * Emerald false coral 0 0 19 Acropora sp. * Staghorn coral 0 0 115 Acropora humilis * Staghorn coral 0 0 1 Acropora yongei * Staghorn coral 0 0 2 Montipora sp. * Montipora coral 0 0 5 Montipora capricornis * Coral 0 0 5 Montipora confusa * Encrusting coral 0 0 22 Montipora danae * Coral 0 0 23 Montipora digitata * Finger coral 0 0 6 Montipora foliosa * Hard coral 0 0 10 Montipora hodgsoni * Coral 0 0 2 Pocillopora sp. * Cauliflower coral 0 0 27 Seriatopora hystrix * Bird nest coral 0 0 8 Stylophora sp. * Cauliflower coral 0 0 1 Stylophora pistillata * Pink cauliflower coral 0 0 23 Catalaphyllia jardinei * Elegance coral 0 0 4 Euphyllia ancora * Crescent coral 0 0 4 Euphyllia glabrescens * Joker's cap coral 0 0 2 Euphyllia paradivisa * Branching frog spawn 0 0 3 Euphyllia paraancora * Branching hammer coral 0 0 3 Euphyllia yaeyamaensis * Crescent coral 0 0 4 Plerogyra sinuosa * Bubble coral 0 0 1 Duncanopsammia axifuga + Coral 0 0 2 Tubastraea sp. -

Brochure Highlight Those Impressive Russia
2019 44 years and counting The products and services listed Join us on Facebook, follow us on Instagram or visit our web site to become one Table of Contents in advertisements are offered and of our growing number of friends who receive regular email updates on conditions Alaska . 4 provided solely by the advertiser. and special big game hunt bargains. Australia . 38 www.facebook.com/NealAndBrownleeLLC Neal and Brownlee, L.L.C. offers Austria . 35 Instagram: @NealAndBrownleeLLC no guarantees, warranties or Azerbaijan . 31 recommendations for the services or Benin . 18 products offered. If you have questions Cameroon . 19 related to these services, please contact Canada . 6 the advertiser. Congo . 20 All prices, terms and conditions Continental U .S . 12 are, to the best of our knowledge at the Ethiopia . 20 time of printing, the most recent and Fishing Alaska . 42 accurate. Prices, terms and conditions Fishing British Columbia . 41 are subject to change without notice Fishing New Zealand . 42 due to circumstances beyond our Kyrgyzstan . 31 control. Jeff C. Neal Greg Brownlee Trey Sperring Mexico . 14 Adventure travel and big game 2018 was another fantastic year for our company thanks to the outfitters we epresentr and the Mongolia . 32 hunting contain inherent risks and clients who trusted us. We saw more clients traveling last season than in any season in the past, Mozambique . 21 dangers by their very nature that with outstanding results across the globe. African hunting remained strong, with our primary Namibia . 22 are beyond the control of Neal and areas producing outstanding success across several countries. Asian hunting has continued to be Nepal . -

Mixed-Species Exhibits with Pigs (Suidae)
Mixed-species exhibits with Pigs (Suidae) Written by KRISZTIÁN SVÁBIK Team Leader, Toni’s Zoo, Rothenburg, Luzern, Switzerland Email: [email protected] 9th May 2021 Cover photo © Krisztián Svábik Mixed-species exhibits with Pigs (Suidae) 1 CONTENTS INTRODUCTION ........................................................................................................... 3 Use of space and enclosure furnishings ................................................................... 3 Feeding ..................................................................................................................... 3 Breeding ................................................................................................................... 4 Choice of species and individuals ............................................................................ 4 List of mixed-species exhibits involving Suids ........................................................ 5 LIST OF SPECIES COMBINATIONS – SUIDAE .......................................................... 6 Sulawesi Babirusa, Babyrousa celebensis ...............................................................7 Common Warthog, Phacochoerus africanus ......................................................... 8 Giant Forest Hog, Hylochoerus meinertzhageni ..................................................10 Bushpig, Potamochoerus larvatus ........................................................................ 11 Red River Hog, Potamochoerus porcus ............................................................... -

Chapter 11A Structuring Herbivore Communities
CHAPTER 11A STRUCTURING HERBIVORE COMMUNITIES The role of habitat and diet SIPKE E. VAN WIEREN AND FRANK VAN LANGEVELDE Resource Ecology Group, Wageningen University, P.O. Box 47, 6700 AA Wageningen, The Netherlands E-mail: [email protected] Abstract. This chapter tries to address the question “Why are there so many species?” with a focus on the diversity of herbivore species. We review several mechanisms of resource specialisation between herbivore species that allow coexistence, ranging from diet specialisation, habitat selection to spatial heterogeneity in resources. We use the ungulate community in Kruger National Park to illustrate approaches in niche differentiation. The habitat overlap of the ungulate species is analysed, continued with the overlap in diet and the spatial heterogeneity in resources. This focus on the constraints on species’ exclusive resources is a useful tool for understanding how competitive interactions structure communities and limit species diversity. In explaining community structure of mobile animals, we argue that the existence of exclusive resources governed by spatial heterogeneity plays an important role. Trade- offs between food availability and quality, food availability and predation risk, or food and abiotic conditions (different habitat types) may constrain competitive interactions among mobile animals and allow the existence of exclusive resources. We propose that body mass of the animals considered is crucial here as animals with different body mass use different resources and perceive spatial heterogeneity in resources differently. A functional explanation of the role of body mass in the structuring of communities is still lacking while the study of how much dissimilarity is minimally needed to permit coexistence between strongly overlapping species is still in its infancy. -
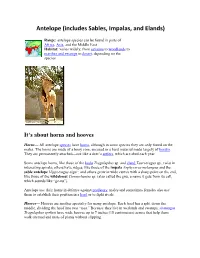
Antelope (Includes Sables, Impalas, and Elands)
Antelope (includes Sables, Impalas, and Elands) Range: antelope species can be found in parts of Africa, Asia, and the Middle East Habitat: varies widely, from savanna to woodlands to marshes and swamps to desert, depending on the species It’s about horns and hooves Horns— All antelope species have horns, although in some species they are only found on the males. The horns are made of a bony core, encased in a hard material made largely of keratin. They are permanently attached—not like a deer’s antlers, which are shed each year. Some antelope horns, like those of the kudu Tragelaphus sp. and eland Taurotragus sp., twist in interesting spirals; others have ridges, like those of the impala Aephyceros melampus and the sable antelope Hippotragus niger; and others grow in wide curves with a sharp point on the end, like those of the wildebeest Connochaetes sp. (also called the gnu, a name it gets from its call, which sounds like “ge-nu”). Antelope use their horns in defense against predators; males and sometimes females also use them to establish their position in a herd or to fight rivals Hooves— Hooves are another specialty for many antelope. Each hoof has a split down the middle, dividing the hoof into two “toes.” Because they live in wetlands and swamps, sitatungas Tragelaphus spekeii have wide hooves up to 7 inches (18 centimeters) across that help them walk on mud and mats of plants without slipping. Nile lechwes Kobus magaceros, which also live in swampy areas, have long, pointed hooves to give them sure footing in the water.