Cutaneous Manifestations of Endocrine Disorders
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Chapter 3 Bacterial and Viral Infections
GBB03 10/4/06 12:20 PM Page 19 Chapter 3 Bacterial and viral infections A mighty creature is the germ gain entry into the skin via minor abrasions, or fis- Though smaller than the pachyderm sures between the toes associated with tinea pedis, His customary dwelling place and leg ulcers provide a portal of entry in many Is deep within the human race cases. A frequent predisposing factor is oedema of His childish pride he often pleases the legs, and cellulitis is a common condition in By giving people strange diseases elderly people, who often suffer from leg oedema Do you, my poppet, feel infirm? of cardiac, venous or lymphatic origin. You probably contain a germ The affected area becomes red, hot and swollen (Ogden Nash, The Germ) (Fig. 3.1), and blister formation and areas of skin necrosis may occur. The patient is pyrexial and feels unwell. Rigors may occur and, in elderly Bacterial infections people, a toxic confusional state. In presumed streptococcal cellulitis, penicillin is Streptococcal infection the treatment of choice, initially given as ben- zylpenicillin intravenously. If the leg is affected, Cellulitis bed rest is an important aspect of treatment. Where Cellulitis is a bacterial infection of subcutaneous there is extensive tissue necrosis, surgical debride- tissues that, in immunologically normal individu- ment may be necessary. als, is usually caused by Streptococcus pyogenes. A particularly severe, deep form of cellulitis, in- ‘Erysipelas’ is a term applied to superficial volving fascia and muscles, is known as ‘necrotiz- streptococcal cellulitis that has a well-demarcated ing fasciitis’. This disorder achieved notoriety a few edge. -

Pattern of Cutaneous Tuberculosis Among Children and Adolescent
Bangladesh Med Res Counc Bull 2012; 38: 94-97 Pattern of cutaneous tuberculosis among children and adolescent Sultana A1, Bhuiyan MSI1, Haque A2, Bashar A3, Islam MT4, Rahman MM5 1Dept. of Dermatology, Bangabandhu Sheikh Mujib Medical University (BSMMU), Dhaka, 2Dept. of Public health and informatics, BSMMU, Dhaka, 3SK Hospital, Mymensingh Medical College, Mymensingh, 4Dept. of Physical Medicine and Rehabilitation, BSMMU, Dhaka, 5Dept. of Dermatology, National Medical College, Dhaka. Email: [email protected] Abstract Cutaneous tuberculosis is one of the most subtle and difficult diagnoses for dermatologists practicing in developing countries. It has widely varied manifestations and it is important to know the spectrum of manifestations in children and adolescent. Sixty cases (age<19 years) of cutaneous tuberculosis were included in this one period study. The diagnosis was based on clinical examination, tuberculin reaction, histopathology, and response to antitubercular therapy. Histopahology revealed 38.3% had skin tuberculosis and 61.7% had diseases other than tuberculosis. Among 23 histopathologically proved cutaneous tuberculosis, 47.8% had scrofuloderma, 34.8% had lupus vulgaris and 17.4% had tuberculosis verrucosa cutis (TVC). Most common site for scrofuloderma lesions was neck and that for lupus vulgaris and TVC was lower limb. Cutaneous tuberculosis in children continues to be an important cause of morbidity, there is a high likelihood of internal involvement, especially in patients with scrofuloderma. A search is required for more sensitive, economic diagnostic tools. Introduction of Child Health (BICH) and Institute of Diseases of Tuberculosis (TB), an ancient disease has affected Chest and Hospital (IDCH) from January to humankind for more than 4,000 years1 and its December 2010. -

Disseminated Mycobacterium Tuberculosis with Ulceronecrotic Cutaneous Disease Presenting As Cellulitis Kelly L
Lehigh Valley Health Network LVHN Scholarly Works Department of Medicine Disseminated Mycobacterium Tuberculosis with Ulceronecrotic Cutaneous Disease Presenting as Cellulitis Kelly L. Reed DO Lehigh Valley Health Network, [email protected] Nektarios I. Lountzis MD Lehigh Valley Health Network, [email protected] Follow this and additional works at: http://scholarlyworks.lvhn.org/medicine Part of the Dermatology Commons, and the Medical Sciences Commons Published In/Presented At Reed, K., Lountzis, N. (2015, April 24). Disseminated Mycobacterium Tuberculosis with Ulceronecrotic Cutaneous Disease Presenting as Cellulitis. Poster presented at: Atlantic Dermatological Conference, Philadelphia, PA. This Poster is brought to you for free and open access by LVHN Scholarly Works. It has been accepted for inclusion in LVHN Scholarly Works by an authorized administrator. For more information, please contact [email protected]. Disseminated Mycobacterium Tuberculosis with Ulceronecrotic Cutaneous Disease Presenting as Cellulitis Kelly L. Reed, DO and Nektarios Lountzis, MD Lehigh Valley Health Network, Allentown, Pennsylvania Case Presentation: Discussion: Patient: 83 year-old Hispanic female Cutaneous tuberculosis (CTB) was first described in the literature in 1826 by Laennec and has since been History of Present Illness: The patient presented to the hospital for chest pain and shortness of breath and was treated for an NSTEMI. She was noted reported to manifest in a variety of clinical presentations. The most common cause is infection with the to have redness and swelling involving the right lower extremity she admitted to having for 5 months, which had not responded to multiple courses of antibiotics. She acid-fast bacillus Mycobacterium tuberculosis via either primary exogenous inoculation (direct implantation resided in Puerto Rico but recently moved to the area to be closer to her children. -

A Case of Lupus Vulgaris Carmen D
Symmetrically Distributed Orange Eruption on the Ears: A Case of Lupus Vulgaris Carmen D. Campanelli, BS, Wilmington, Delaware Anthony F. Santoro, MD, Philadelphia, Pennsylvania Cynthia G. Webster, MD, Hockessin, Delaware Jason B. Lee, MD, New York, New York Although the incidence and morbidity of tuberculo- sis (TB) have declined in the latter half of the last decade in the United States, the number of cases of TB (especially cutaneous TB) among those born outside of the United States has increased. This discrepancy can be explained, in part, by the fact that cutaneous TB can have a long latency period in those individuals with a high degree of immunity against the organism. In this report, we describe an individual from a region where there is a rela- tively high prevalence of tuberculosis who devel- oped lupus vulgaris of the ears many years after arrival to the United States. utaneous tuberculosis (TB) is a rare manifes- tation of Mycobacterium tuberculosis infection. C Scrofuloderma, TB verrucosa cutis, and lupus vulgaris (LV) comprise most of the cases of cutaneous TB. All 3 are rarely encountered in the United States. During the last several years, the incidence of TB has declined in the United States, but the incidence of these 3 types of cutaneous TB has increased in foreign-born individuals. This discrepancy can be ex- plained, in part, by the fact that TB can have a long latency period, especially in those individuals with a Figure 1. Orange plaques and nodules on the right ear. high degree of immunity against the organism. Indi- viduals from regions where there is a high prevalence Case Report of TB may develop cutaneous TB many years after ar- A 71-year-old man from the Philippines presented rival to the United States, despite screening protocol with an eruption on both ears that had existed for when they enter the United States. -

Nontuberculous Mycobacterial Skin Infection: Cases Report And
วารสารวิชาการสาธารณสุข Journal of Health Science ปี ท ี � �� ฉบับที� � พฤศจิกายน - ธันวาคม ���� Vol. 23 No. 6, November - December 2014 รายงานผู้ป่วย Case Report Nontuberculous Mycobacterial Skin Infection: Cases Report and Problems in Diagnosis and Treatment Jirot Sindhvananda, M.D., Preya Kullavanijaya, M.D., Ph.D., FRCP (London) Institute of Dermatology, Department of Medical Services, Ministry of Public Health, Thailand Abstract Nontuberculous mycobacteria (NTM) are infrequently harmful to humans but their incidence increases in immunocompromised host. There are 4 subtypes of NTM; among them M. marinum is the most common pathogen to human. Clinical manifestation of NTM infection can mimic tuberculosis of skin. Therefore, supportive evidences such as positive acid-fast bacilli smear, characteristic histopathological finding and isolation of organism from special method of culture can help to make the definite diagnosis. Cases of NTM skin infection were reported with varying skin manifestations. Even patients responsed well with many antimicrobial agents and antituberculous drug, some difficult and recalcitrant cases have partial response especially in M. chelonae infected-cases. Kay words: nontuberculous mycobacteria, M. chelonae, skin infection, treatment Introduction were once termed as anonymous, atypical, tubercu- Nontuberculous mycobacteria (NTM) are infre- loid, or opportunistic mycobacteria that are infre- quently harmful to humans but their incidence in- quently harmful to humans(1-4). Until recently, there creases in immunocompromised host. There are 4 were increasing coincidences of NTM infections with subtypes of NTM; and the subtype M. marinum is the a number of immunocompromised and AIDS cases. most common pathogen to human(1). Clinical mani- The diagnosis of NTM infection requires a high festation of NTM infection can mimic tuberculosis of index of suspicion. -

Lupus Vulgaris with Unusual Involvement
LUPUS VULGARIS WITH UNUSUAL INVOLVEMENT Cihangir Aliağaoğlu1, Mustafa Atasoy2, Ümran Yıldırım3, R. İsmail Engin2, Handan Timur2 Düzce University, Faculty of Medicine, Departments of Dermatology and Pathology3, Düzce, Atatürk University, Faculty of Medicine, Department of Dermatology2, Erzurum, Turkey Lupus vulgaris is the most encountered form of cutaneous tuberculosis, and the most common site of involvement is the head and neck. In our lupus vulgaris cases, the lesions were located in throcal area in one case and gluteal area in the other. Ziehl-Neelsen and periodic acid-Schiff stains did not demonstrate any acid-fast bacilli. Culture did not grow mycobacterium tuberculosis except in case 1. PPD was strongly positive in all of the cases. Lesions of lupus vulgaris improved after anti-tuberculotic threrapy. Key words: Lupus vulgaris, unusual involvement Eur J Gen Med 2007; 4(3):135-137 INTRODUCTION gave an apple-jelly appearance. The systemic Lupus vulgaris (LV) is usually the result examination was normal. Lymph nodes were of dissemination from an endogenous focus not palpable. No BCG scar was visible. The during a period of lowered resistance and entire dermis was composed of non-caseous mycobacterium tuberculous bacillemia in a granulomatous inflammation which contains previously sensitized host with a strongly epitheloid histiocytes, lymphocytes, and positive delayed hypersensitivity to tuberculin large numbers of Langhans type giant cells (1). LV is often located on the face. Other sites (Figure 1B). A Mantoux test was positive of predilection are the nose, ears, chin, neck, with erythema and induration of 18 mm after and, rarely, extremities, buttock and trunk. 48 hours. Mycobacterium tuberculosis was It is more common in females than in males, cultured from the biopsy specimen. -

Delayed Granulomatous Lesion at the Bacillus Calmette-Gue´Rin Vaccination Site
302 Letters to the Editor baseline warts (imiquimod 11% vs. vehicle 6%; p = 0.488), 2. Buetner KR, Spruance SL, Hougham AJ, Fox TL, Owens ML, more imiquimod-treated patients experienced a 50% reduc- Douglas JM Jr. Treatment of genital warts with an immune- tion in baseline wart area (38% vs. 14%; p = 0.013). Use of response modi er (imiquimod). J Am Acad Dermatol 1998; 32: imiquimod was not associated with any changes in laboratory 230–239. values, including CD4 count. It was not associated with any 3. Beutner KR, Tyring SK, Trofatter KF, Douglas JM, Spruance S, adverse drug-related events, and no exacerbation of HIV/AIDS Owens ML, et al. Imiquimod, a patient-applied immune-response was attributed to the use of imiquimod. However, it appeared modi er for treatment of external genital warts. Antimicrob Agents Chemother 1998; 42: 789–794. that topical imiquimod was still less eVective at achieving total 4. Tyring SK, Arany I, Stanley MA, Tomai MA, Miller RL, clearance than in the studies with HIV-negative patients, which Smith MH, et al. A randomized, controlled, molecular study of is most likely a re ection of the impaired cell-mediated condylomata acuminata clearance during treatment with imiqui- immunity seen in the HIV-positive population (8). mod. J Infect Dis 1998; 178: 551–555. There has also been a report of improved success when topical 5. Arany I, Tyring SK, Stanley MA, Tomai MA, Miller RL, imiquimod was combined with more traditional destructive Smith MH, et al. Enhancement of the innate and cellular immune therapy for HPV infection in HIV-positive patients, particularly response in patients with genital warts treated with topical imiqui- in the setting of the use of highly-active antiretroviral therapy mod cream 5%. -

Lupus Vulgaris of the External Nose in a Paediatric Patient: a Case Report from Muhimbili National Hospital, Tanzania
Global Journal of Otolaryngology ISSN 2474-7556 Case Report Glob J Otolaryngol Volume 19 Issue 4 - March 2019 Copyright © All rights are reserved by Zephania Saitabau Abraham DOI: 10.19080/GJO.2019.19.556019 Lupus Vulgaris of the External Nose in a Paediatric Patient: A Case Report from Muhimbili National Hospital, Tanzania Zephania Saitabau Abraham1*, Daudi Ntunaguzi2 Enica Richard Massawe2 and Aveline Aloyce Kahinga2 1Department of Surgery, University of Dodoma-College of Health Sciences, Tanzania 2Department of Otorhinolaryngology-Muhimbili University of Health and Allied Sciences, Tanzania Submission: March 07, 2019; Published: March 13, 2019 *Corresponding author: Zephania Saitabau Abraham, Department of Surgery, University of Dodoma-College of Health Sciences, Tanzania Abstract histopathologically.Lupus vulgaris isOn the local most examination, common form the ofpatient cutaneous had irregularly tuberculosis bordered, which usually well demarcated, occurs in patients whitish who to reddish have been lesion previously on her external sensitized nose. to Mycobacterium tuberculosis. We present a case of a 4-year-old girl who was diagnosed to have lupus vulgaris clinically and was then confirmed histopathologicalThe histopathological basis examination so as to avoid showed its destructive many dermal consequences stromal granulomaswhich are mainly of epithelioid erosion of cells, the externalmany multinucleated nose, nasal cavity giant and cells the of face Langhans and in raretype. occasions, This case reportpossible is thereforedevelopment -

Multifocal Cystic Bone Tuberculosis with Lupus Vulgaris and Lymphadenitis
INDIAN PEDIATRICS VOLUME 34-MAY 1997 via cavernous sinus and intracranial propa- CT or an MRI should be done as the inves- gation of periorbital cellulitis. This was not tigation of choice to differentiate between required in our case due to probably insti- an inflammatory lesion and a tumor. tution of appropriate antibiotic therapy. It may be of merit in severe or late diagnosed REFERENCES cases. Staphylococcal colonization of na- 1. Besley G, Minns RA. Disorders of the cen- sopharynx can lead to ethmoiditis in tral nervous system. In: Forfar and immunocompromized or susceptible child, Ameil's Textbook of Pediatrics, 4th edn. with rapid progress locally and hematoge- Eds. Brown JK, Campbell AGM, Mclntosh nous seeding as in our case. A variety of or- N. Edinburgh, Churchill Livingstone, ganisms other than staphylococcus can 1992, pp 898-901. cause this infection, which is dangerous be- 2. Haynes RE, Cramblett HG. Acute cause it may be complicated by retrobulbar ethmoiditis: Its relationship to orbital cellulitis. Am J Dis Child 1967; 114: 261- abscess and cavernous sinus infection and 267. thrombosis. Treatment should be started 3. Moranne JE, Estorunet B, Adrien A, early with intravenous antibiotics. Also, Seurat MC, Barois A. Staphylococcus, the concomitant staphylococcal foci should be most frequent agent of serious complica- looked for and dealt with surgically if nec- tions of acute sinusitis in children: 5 cases. essary. If in any doubt, a contrast enhanced Ann Intern Med 1982; 133: 462-467. Multifocal Cystic Bone Tuberculosis Rarer still is its association with skin tuber- with Lupus Vulgaris and culosis and lymphadenopathy. -

Atypical Mycobacterial Cutaneous Infections in Hong Kong
ORIGINAL ARTICLE CME MH Ho CK Ho Atypical mycobacterial cutaneous LY Chong infections in Hong Kong: 10-year retrospective study !"#$%&'()*+,-./01 ○○○○○○○○○○○○○○○○○○○○○○○○○○○○○○○○○○○○○○○ Objective. To review the epidemiology of atypical mycobacterial cutaneous infection in Hong Kong. Design. Retrospective study. Setting. Social Hygiene Service (Dermatology Division), the largest dermato- logical referral centre in Hong Kong. Patients. Patients with a diagnosis of atypical mycobacterial cutaneous infec- tion based on clinical features, histopathology, with or without a positive culture during the period 1993 to 2002. Main outcome measures. Epidemiological data, clinical features, histology, microbiological investigation, and treatment response. Results. Of 345 394 dermatological cases presented over the 10-year period, 33 (0.0096%) cases (19 male, 14 female) of atypical mycobacterial cutaneous infection were diagnosed. The most common type of infection was caused by Mycobacterium marinum (n=17, 51.5%), followed by Mycobacterium avium-intracellulare (n=3, 9.1%) and Mycobacterium chelonae (n=2, 6.1%). The upper limb, especially the hands and fingers, was the most common (69.7%) site of involvement. Tissue culture was positive in 18 (54.5%) cases. All biopsies showed granulomatous histology. Thirty-two patients received treatment and 31 responded. Twenty-six were treated with oral tetracycline group of antibiot- ics (minocycline, doxycycline, tetracycline). The duration of treatment ranged from 8 to 54 weeks (mean, 24 weeks). Mild transient adverse effects to treatment were reported in six cases. Conclusion. Atypical mycobacterial infection is rare in Hong Kong. Because of the low sensitivity of traditional culture techniques, atypical mycobacterial infec- tion may be underdiagnosed if only culture-confirmed cases are included. -

Chapter 1: Bacterial Infections
Atlas of Paediatric HIV Infection CHAPTER 1: BACTERIAL INFECTIONS Staphylococcal Infections A high percentage of HIV-infected persons are nasal carriers of Staphylococcus aureus, hence the high rate of infection in this population. Impetigo Description: Impetigo is a superficial bacterial skin infection characterised by flaccid pustules and honey-coloured crust. It usually begins as a small painful erythematous papule. Aetiology: The most common implicated organism is Staphylococcus aureus, although group A beta- hemolytic streptococcus (Streptococcus pyogenes) has been implicated in some cases. Clinical presentation: Impetigo can be bullous and non-bullous, usually on the face and extremities. Primary impetigo presents as erythematous plaques with or without thin-walled vesicles that break down leaving characteristic yellow crust. Secondary impetigo can occur in other dermatoses e.g. eczema. Epidemiology: Impetigo is common in children especially those aged 2-5 years and prevalence of 15 - 25% has been reported in the tropics. It is transmitted by contact with infected skin. Diagnosis: Diagnosis is usually clinical but a Gram stain and culture may be required to confirm diagnosis when there is extensive disease. Treatment: This should be guided by local antibiotics sensitivity testing but in mild and localized infection, first-line topical antibiotics like mupirocin, bacitracin or fusidic acid for 7-10 days are effective. If the infection is widespread, severe or is associated with lymphadenopathy, oral penicillins (flucloxacillin) or macrolides (erythromycin) if patient is allergic to penicillins, are indicated for 7-10 days. Parenteral antibiotics may be required if impetigo is diagnosed in a very sick child. Complications: Cellulitis, osteomyelitis, staphylococcal scalded skin syndrome, and acute post- streptococcal glomerulonephritis can occur. -
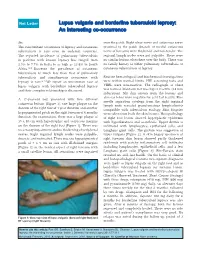
Lupus Vulgaris and Borderline Tuberculoid Leprosy: an Interesting Co-Occurrence
Net Letter Lupus vulgaris and borderline tuberculoid leprosy: An interesting co-occurrence Sir, over the patch. Right ulnar nerve and cutaneous nerve The concomitant occurrence of leprosy and cutaneous proximal to the patch (branch of medial cutaneous tuberculosis is rare even in endemic countries. nerve of forearm) were thickened and non-tender. The The reported incidence of pulmonary tuberculosis regional lymph nodes were not palpable. There were in patients with known leprosy has ranged from no similar lesions elsewhere over the body. There was 2.5% to 7.7% in India to as high as 13.4% in South no family history of either pulmonary tuberculosis or Africa.[1-3] However the prevalence of cutaneous cutaneous tuberculosis or leprosy. tuberculosis is much less than that of pulmonary tuberculosis and simultaneous occurrence with Routine hematological and biochemical investigations leprosy is rare.[4-7] We report an uncommon case of were within normal limits. HIV screening tests and lupus vulgaris with borderline tuberculoid leprosy VDRL were non-reactive. The radiograph of chest and their complex relationship is discussed. was normal. Mantoux test was hyper-reactive (18 mm induration). Slit skin smears from the lesions and also ear lobes were negative for acid fast bacilli. Fine A 17-year-old boy presented with two different needle aspiration cytology from the right inguinal cutaneous lesions [Figure 1], one large plaque on the lymph node revealed granulomatous lymphadenitis dorsum of the right foot of 1 year duration and another compatible with tuberculous etiology. Skin biopsies hypopigmented patch on the right forearm of 6 months were taken from both the skin lesions.