Carbon Sequestration Potential of Wetlands Across Punjab and Possible Climate Mitigation Strategy
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
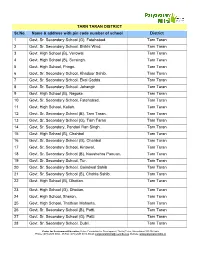
TARN TARAN DISTRICT Sr.No. Name & Address With
TARN TARAN DISTRICT Sr.No. Name & address with pin code number of school District 1 Govt. Sr. Secondary School (G), Fatehabad. Tarn Taran 2 Govt. Sr. Secondary School, Bhikhi Wind. Tarn Taran 3 Govt. High School (B), Verowal. Tarn Taran 4 Govt. High School (B), Sursingh. Tarn Taran 5 Govt. High School, Pringri. Tarn Taran 6 Govt. Sr. Secondary School, Khadoor Sahib. Tarn Taran 7 Govt. Sr. Secondary School, Ekal Gadda. Tarn Taran 8 Govt. Sr. Secondary School, Jahangir Tarn Taran 9 Govt. High School (B), Nagoke. Tarn Taran 10 Govt. Sr. Secondary School, Fatehabad. Tarn Taran 11 Govt. High School, Kallah. Tarn Taran 12 Govt. Sr. Secondary School (B), Tarn Taran. Tarn Taran 13 Govt. Sr. Secondary School (G), Tarn Taran Tarn Taran 14 Govt. Sr. Secondary, Pandori Ran Singh. Tarn Taran 15 Govt. High School (B), Chahbal Tarn Taran 16 Govt. Sr. Secondary School (G), Chahbal Tarn Taran 17 Govt. Sr. Secondary School, Kirtowal. Tarn Taran 18 Govt. Sr. Secondary School (B), Naushehra Panuan. Tarn Taran 19 Govt. Sr. Secondary School, Tur. Tarn Taran 20 Govt. Sr. Secondary School, Goindwal Sahib Tarn Taran 21 Govt. Sr. Secondary School (B), Chohla Sahib. Tarn Taran 22 Govt. High School (B), Dhotian. Tarn Taran 23 Govt. High School (G), Dhotian. Tarn Taran 24 Govt. High School, Sheron. Tarn Taran 25 Govt. High School, Thathian Mahanta. Tarn Taran 26 Govt. Sr. Secondary School (B), Patti. Tarn Taran 27 Govt. Sr. Secondary School (G), Patti. Tarn Taran 28 Govt. Sr. Secondary School, Dubli. Tarn Taran Centre for Environment Education, Nehru Foundation for Development, Thaltej Tekra, Ahmedabad 380 054 India Phone: (079) 2685 8002 - 05 Fax: (079) 2685 8010, Email: [email protected], Website: www.paryavaranmitra.in 29 Govt. -

Urban Wastewater: Livelihoods, Health and Environmental Impacts in India: the Case of the East Calcutta Wetlands
Urban Wastewater: Livelihoods, Health and Environmental Impacts in India: The Case of the East Calcutta Wetlands Principal Investigator Gautam Gupta Department of Economics Jadavpur University Kolkata 700 032 Objectives: Identify the livelihood options in and around East Calcutta Wetlands based on use of Urban Wastewater Estimate the value of Direct benefits derived from the use of Urban Wastewater by the stakeholders in ECW Health Impacts of Urban Wastewater on Stake holders Environmental impact of ECW on Stakeholders Geographical Features of ECW The wetlands to the East of Kolkata, well known over the world, situated in between 22°25´- 22°40´ N latitude and 88°20´-88°35´E longitude and covering the area of about 12,500 ha. It has a hot and humid monsoon climate governed by the Himalayas in the north and the Bay of Bengal in the south January is the coolest month with temperatures ranging between 10°C to 20°C while May experiences maximum temperature ranging between 30°C and 40°C Average relative humidity is high between 70 percent and 90 percent approximately. Average annual rainfall is about 1582 mm and is mainly concentrated in the months of June, July, August and September. Hydrology of East Calcutta Wetlands Sewage is largely water but it contains organic and inorganic solids in dissolved and suspended forms. Major bacteria accompanying these solids are coliform A major problem in the hydrology of East Calcutta Wetlands, is arsenic. The percentage of arsenic which is considered safe for consumption is 10mg/litre as estimated by WHO. However in the northern limits of greater Kolkata, in the areas like Bhangar, Kharibari, Rajarhat, Bishnupur I and II, Gangra, Mahisbathan II the levels of arsenic has been found to be 10-15mg/litre. -

Punjab Police GK
Punjab GK Most Important MCQs of Punjab Police Exams 1. What is the literal meaning of the name Punjab? C) Dr. Jaswinder Singh A) Land of five rivers D) Kirpal Kazak B) Land of seven rivers C) Area near Mount Abu Answer: D) Kingdom of five Pandavs Gurdev Singh Rupana won the Sahitya Akademi Award Answer: for Punjabi language in the year 2020. It is the highest The correct Answer is Land of five rivers. The name literary award in India and he got this award for his book Punjab is made of two words Punj (Five) + Aab (Water) of short stories Aam Khass (ਆਮ-ਖ਼ਾਸ). i.e. land of five rivers and these five rivers of Punjab are Satluj, Beas, Ravi, Chenab & Jhelum. 6. What was the theme of Punjab's tableau in the Repulic Day Parade 2021 at New Delhi? 2. Which city of Punjab is famous for manufacturing of A) Jallianwal Bagh Massacre sports goods? B) Martyrdom of Guru Teg Bahadur Ji A) Ludhiana C) Sangat and Pangat B) Patiala D) Maharaja Ranjit Singh C) Batala Answer: D) Jalandhar In the Republic Day Parade 2021 at New Delhi, the Answer: theme of Punjab's tableau was the martyrdom of Shri Jalandhar city is famous for manufacturing of sports Guru Teg Bahadur ji. Guru ji took up the cause of goods. The sports items are supplied all through India Kashmiri pandits, who were facing religious persecution and also exported to many other countries. and conversions to Islam by mughal emperor Aurangzeb and was martyred in 1675 at Chandni Chowk, Delhi. -

Catla Catla, Labeo Rohita and Cirrhinus Mrigala) in Morvan Dam (Neemuch District), M.P., India
World Applied Sciences Journal 39 (1): 37-43, 2021 ISSN 1818-4952 © IDOSI Publications, 2021 DOI: 10.5829/idosi.wasj.2021.37.43 Elucidation of Length-Weight Relationship and Condition Factor for Indian Major Carps (Catla catla, Labeo rohita and Cirrhinus mrigala) in Morvan Dam (Neemuch District), M.P., India 12Resham Rajput and Nageshwar Wast 1Department of Zoology, Govt. Girls College, Khandwa, M.P., India 2PG Department of Zoology, J.P. University, Chapra, Bihar, India Abstract: A study has been carried out to elucidate the length-weight relationship and condition factor of Indian major carps (Catla catla, Labeo rohita and Cirrhinus mrigala) in Morvan Dam (Neemuch District), during September to November month of the two consecutive years (2018 and 2019) to understand the present status of the fish. The maximum mean value of total lengths were analyzed for Catla catla, Labeo rohita and Cirrhinus mrigala as 85.20, 48.30 and 46.00 cm whereas, the maximum mean value of total weight were estimated as 9200, 1350 and 1330 gm respectively during November, 2018. Highest increase in average length were recorded as 39.60% for Labeo rohita during 2018 and lowest as 22.86 % during 2019 for Cirrhinus mrigala however, the percentage (%) increase in average weight were documented highest for Catla catla (49.86%) and lowest for Cirrhinus mrigala (12.15%) during 2019. The Condition Factor (K) for Catla catla, Labeo rohita and Cirrhinus mrigala were noticed in the ranges from 1.4875 to 2.5343, 1.1883 to 2.4142 and 1.3664 to 2.3233, respectively during both the studied years which indicates about the good health condition of fishes in Morvan Dam.The length and weight variables of Catla catla, Labeo rohita and Cirrhinus mrigala exhibited linear relationships which are illustrated by coefficient correlation (r), exponent values ‘b’ (indicates positive allometric growth). -

Village & Townwise Primary Census Abstract, Kapurthala, Part X-A & B, Series-17, Punjab
CENSUS 1971 PARTS X-A & B VILLAGE & TOWN SERIES 17 DIRECTORY PUNJAB VILLAGE & TOWNWISE PRIMARY CENSUS ABSTRACT DISTRICT CENSUS KAPURTHALA HANDBOOK DISTRICT P. L. SONDHI H. S. KWATRA OF THE INDIAN ADMINISTRATIVE SERVICE OF THE PUNJAB CIVil, SERVICE Ex-Officio Director of Census Opemtions Deputy Director of Census Opemtions PUNJAB PUNJAB Motif-- GURDWARA BER SAHIB, SULTANPUR LODHI Gurdwara Be?" Sahib is a renowned place of pilgrimage of the Sikhs. It is situated at Sultanpur Lodhi, 16 miles South of Kapurthala, around a constellation of other Gurdwaras (Sikh Temples) associated with the early life of Guru Nanak Dev. It is n:a,.med after the 'Ber', tree under which Guru Nanak Dev used to meditate. Legend has it that sterile women beget child7'en after takinq leaves of this tree. The old Gu'rdwara was re-constructed by the joint effo'rts of Maharaja Jagatjit Singh of Kapurthala, Maharaja Yadvindra Singh of Patiala and Bhai Arjan Singh of Bagrian. A big fair is held at this Gurdwara on Guru Nanak Dev's birthday. Motif by : J. S. Gill. 15 '40' PUNJAB DISTRICT KAPURTHALA s· KILOIUTRES S o 5 10 15 20 4 8 12 MILES 4 o· 3 " Q TO JUL LlJNDllR <' ~O "'''<, U ""a". I. \.. u .) . 31 DISTRICT 80UNOARV..... POST' TtLEGftAPH OFfiCE "................. P'T TAHSIL BOUNDARY.. _TALlil PRIMARV HEALTH DISTRICT HEADQUARTERS .. CENTRE S IMATERNITY • CHIlD T"HSIL HEADQUARTERS. WELfARE CENTRES ............... - ... $ NATIONAL HIGHWAY .. liECONDARY SCHOOL./COl.LEGE .............•..• , OTHER METAI.LED ROAII.. 45 BROAD GAUGE RAILWAYS WITH STATIOfll. ... RS 4 RIVER .. - CANAL .. UklAII AREA •.. RUT HOUSE .... VILLAQES HAVING POPULATION 5000+ URBAN POPULATION " 50.000 PERSONS 10.000 •.. -

National Report on the Implementation of the Ramsar Convention on Wetlands
NATIONAL REPORT ON THE IMPLEMENTATION OF THE RAMSAR CONVENTION ON WETLANDS National Reports to be submitted to the 10th Meeting of the Conference of the Contracting Parties, Republic of Korea, 28 October – 4 November 2008 Please submit the completed National Report, in electronic (Microsoft Word) format, and preferably by e-mail, to the Ramsar Secretariat by 31 March 2008. National Reports should be sent to: Alexia Dufour, Regional Affairs Officer, Ramsar Secretariat ([email protected]) 1 Introduction & background 1. This Ramsar COP10 National Report Format (NRF) has been approved by the Standing Committee for the Ramsar Convention’s Contracting Parties to complete as their national reporting to the 10th meeting of the Conference of the Contracting Parties of the Convention (Republic of Korea, October/November 2008). 2. Following Standing Committee discussions at its 35th meeting in February 2007, and its Decisions SC35-22, -23 and -24, this COP10 National Report Format has been significantly revised and simplified in comparison with the National Report Formats provided to previous recent COPs. 3. In particular this National Report Format provides a much smaller number (66) of implementation “indicator” questions, compared with the much larger suite of questions on all aspects of national implementation of the Convention’s Strategic Plan 2003-2008 included in previous NRFs. 4. The COP10 NRF indicators include, with the agreement of the Standing Committee (Decision SC35-24), certain indicators specifically requested to be included by the Convention’s Scientific & Technical Review Panel (STRP) and CEPA Oversight Panel, in order to facilitate their information gathering and reporting on key aspects of scientific, technical and CEPA implementation under the Convention. -

And Condition Factor of Labeo Rohita and Cirrhinus Mrigala in Sutiapat
Journal of Entomology and Zoology Studies 2019; 7(1): 420-424 E-ISSN: 2320-7078 P-ISSN: 2349-6800 Studies on length-weight relationship (LWR) and JEZS 2019; 7(1): 420-424 © 2019 JEZS condition factor of Labeo rohita and Cirrhinus Received: 01-11-2018 Accepted: 03-12-2018 mrigala in sutiapat reservoir, Kabirdham, Ritesh Chandrvanshi Chhattisgarh, India Department of Aquaculture College of Fisheries, Central Agriculture University, Lembucherra, Tripura, India Ritesh Chandrvanshi, Rakesh Uraon, Niranjan Sarang and HK Vardia Rakesh Uraon Abstract College of Fisheries, CGKV, This study aimed to estimate the length-weight relationship and characterized the condition factor of Kawardha, Chhattisgarh, India Labeo rohita and Cirrhinus mrigala is one of the most abundant and economically important species in Niranjan Sarang sutiapat reservoir. The Sutiapat reservoir is a medium irrigation reservoir which is located in the Department of Fisheries Kabirdham district of Chhattisgarh. The present study describes the length-weight relationship and Resource Management condition factor of L. rohita and C. mrigala to determine the growth pattern. The fish sampled range College of Fisheries, CGKV, between total length 38.30 cm to 62.00 cm and total weight 850.00 gm to 3000.00 gm for L. rohita and Kawardha, Chhattisgarh, India C. mrigala from 36.50 cm. to 48.00 cm. in total length and 800.00 gm to 1250.00 gm in total weight. The correlation coefficient ‘r’ was found to be significant and observed to be 0.96 and 0.94 in L. rohita and C. HK Vardia mrigala respectively. The experimental fish ranged the length-weight relationship was calculated based Department of Aquaculture on a sample collected during the study period revealed a strong linear relationship between total length College of Fisheries, CGKV, and weight. -

Environmental Science Quiz Q1
ENVIRONMENTAL SCIENCE QUIZ Q1. Punjab’s state aquatic animal is A. Indus River Dolphin B. Otter C. Gharial D. Turtle Q2. Ozone layer lies in which layer of atmosphere? A. Stratosphere B. Exosphere C. Troposphere D. Mesosphere Q3. Which gas is the most abundant in earth’s atmosphere? A. Oxygen B. Nitrogen C. Hydrogen D. Carbon dioxide Q4. The percentage of solar energy utilization by the plants in a food chain is : A. 10% B. 0.01% C. 0.1% D. 1% Q5. Energy flow in an ecosystem follows which of the following progression A. Consumers---->Producers---->Decomposers B. Producers---->Decomposers---->Consumers C. Decomposers---->Consumers---->Producers D. Producers---->Consumers---->Decomposers Q6. COD is A. Chemical Oxide Demand B. Chemical Ozone Demand C. Chemical Oxygen Demand D. None of these Q7. Bir Moti Bagh Wildlife Sanctuary is situated in which district of Punjab? A. Hoshiarpur B. Patiala C. Pathankot D. Gurdaspur Q8. What is the percentage of forest in Punjab? A. 5.28% B. 8.34% C. 6.12% D. 7.21% Q9. On which date is “World Environment Day” celebrated every year to mark “Stockholm Conference” on Human Environment held in Sweden in 1972. A. March 28 B. June 5 C. May 23 D. October 18 Q10. Ozone layer thickness is measured in A. Dobson Units B. Candella C. Melson Units D. Sieverts Q11. Among the following which National Park is famous as “Sairandhri Vanam”? A. Silent Valley National Park B. Rajaji National Park C. Periyar National Park D. Jim Corbett National Park Q12. Montreal protocol is associated with A. -

Ramsar (Wetlands) Sites in India
www.gradeup.co Ramsar (Wetlands) Sites in India What are the Wetlands? • It is a place where the land is covered by salty or fresh water. Swamps, Marshes, ponds, the edge of lake or ocean, river mouths and deltas etc. are the examples of the Wetlands. • Wetlands are one of the most productive ecosystems in the world and essential for human survival. • Wetlands are home to various species of mammals, birds, fishes and invertebrates. They support the cultivation of crops like rice, and also provide ecological services benefiting the human race like water filtration, storm protection, flood control etc. Why are Wetlands are called Ramsar Sites? • In 1971, an international treaty was signed at Ramsar, Iran for the conservation and sustainable use of wetlands. • The mission of the Convention is “the conservation and wise use of all wetlands through local and national actions and international cooperation, as a contribution towards achieving sustainable development throughout the world”. • Ramsar Convention is an only intergovernmental treaty which gives a solid framework to the nations for the conservation and use of wetlands and their resources and helps to protect such unique ecosystems. • It is also known as the “Convention on Wetlands”. It was adopted in the Iranian city of Ramsar on 2nd February 1971 and came into force on 21 December 1975. Facts about Ramsar Sites: • So 2nd February is celebrated as “World Wetlands Day” every year. • Currently, 169 countries are a party to this convention. There are 2289 wetland sites, covering an area around 225399512 hectors, designated under this convention. • The United Kingdom has the most number of sites - 170. -

Water Quality Issues and Challenges in Punjab
iatkc ds ty xq.koRrk eqís vkSj pqukSfr;ka Water Quality Issues and Challenges in Punjab dsanzh; Hkwfe tycksMZ Central Ground Water Board ty lalk/ku ea=kky; Ministry of Water Resources Hkkjr ljdkj Government of India March 2014 vuqØef.kdk v/;k; fooj.k i`"B la- dk;Zdkjh lkjka'k 1 i`"BHkwfe 1 2 ty xq.koRrk vkSj LokLF; laca/kh eqís 3 2-1 ekuo LokLF; ij izHkko 3 2-2 i'kq/ku ij izHkko 4 2-3 ikS/kksa ds fodkl ij izHkko 5 2-4 mn~;ksxksa ij izHkko 5 3 ty vkSj Hkwfe dk mi;ksx 7 3-1 vkd`fr foKku ,oa ty fudklh 7 3-2 tyok;q vkSj o"kkZ 16 3-3 e`nk ,oa lw{e iks"kd 17 3-4 Hkwfe dk mi;ksx 20 3-5 d`f"k mRikndrk 24 3-6 flapkbZ lqfo/kk,a 28 3-7 ty lalk/ku 29 4 ty iznw"k.k vkSj bldk i;kZoj.k 44 4-1 Hkwtfur lanw"k.k 45 4-2 ekuotfur lanw"k.k 48 5 laxBukRed laidZ vkSj fuxjkuh 54 5-1 dsanzh; Hkwfety cksMZ] Hkkjr ljdkj 54 5-2 i;kZoj.k foHkkx] iatkc fo'ofon~;ky;] paMhx<+ 54 5-3 LokLF; vkSj ifjokj dY;k.k foHkkx] iatkc ljdkj 55 5-4 ty vkiwfrZ ,oa LoPNrk foHkkx] iatkc ljdkj 55 5-5 iatkc iznw"k.k fu;a=k.k cksMZ] iatkc ljdkj 56 5-6 lkoZtfud LokLF; vkSj lkeqnkf;d fpfdRlk Ldwy ihthvkbZ,ebZvkj] paMhx<+ 57 5-7 ty lalk/ku vkSj i;kZoj.k funs'kky;] iatkc ljdkj 57 6 ty xq.koRrk :>ku vkSj izHkko 59 6-1 lrgh ty xq.koRrk 59 6-2 Hkwty xq.koRrk 69 6-3 vfHkdj.k&okj fo'ks"k v/;;u 81 6-4 ty xq.koRrk dh rqyuk esa fodkl dk izHkko 109 7 Toyar eqísa 116 8 ty xq.koRrk izca/ku dk;Z uhfr 124 8-1 izca/ku fodYi 126 8-2 fofu;eu vkSj uhfr dk;Z <kapk 131 9 Hkfo"; dh vksj vxzlj 137 vuqca/k 140 TABLE OF CONTENTS CHAPTER DESCRIPTION PAGE NO. -

Wetlands of Majuli: the Second Largest River Island of the World Majuli, the Largest Human Inhabited River Island of Assam
HHimalayanimalayan EEcologycology ISSN: 2277-9000 Vol. 11 (3), 2014 Inside the issue ... Securing the future of Himalayan Wetlands The wetlands of Indian Himalayan Region (IHR)..... Page 1 Wetlands of Majuli: The second largest river island of the world Majuli, the largest human inhabited river island of Assam..... Page 3 Aquaculture and Ecotourism Potential in Arunachal Pradesh: Mehao and Ganga lakes Sharma Subrat credit: Photo Arunachal Pradesh lying within the high An arial view of Loktak Lake precipitation zone is..... Page 4 Loktak Lake: A fresh water lake of Securing the future of Himalayan wetlands International Importance in NE India Loktak lake located in the Bishnupur..... he wetlands of Indian Himalayan Region February, marks the date of the adoption of the Page 5 T(IHR) scattered in different geographical Convention on Wetlands on 2 February, 1971 regions from high altitude cold arid zone of Ladakh in Iran. Deepor Beel: The Ramsar Site of Assam to the flood plains of Brahmaputra, are mosaic of The growing concern about the Deepor Beel Wildlife Sanctuary and the Ramsar site..... varying sizes ranging from lentic to lotic habitats. conservation of wetland biodiversity has led They have a great role to play in preserving the the search for more eco-friendly, sustainable Page 6 earth’s fragile eco-system and are regarded as direct and more effective as well as economic Lakes of Kumaun in Uttarakhand: or indirect life supporting systems for millions of strategies. Identification of the key drivers Temporal variation in water..... living beings having great economic, aesthetic and of wetland change and adoption of suitable Uttarakhand state comprises two major scientific importance. -

Ramsar-Sites-In-India.Pdf
Ramsar Sites in India - List of Ramsar Sites Ramsar Sites are the wetlands that have international importance. The term was coined when the International Treaty for the Conservation and Sustainable Use of Wetlands was signed at a city of Iran called Ramsar in 1971. The topic, 'Ramsar Sites of India' is important for the upcoming IAS Exam as recently Sambhar Lake had been in the news for its deterioration over salt mining. Sambhar Lake is a Ramsar Site in India. Hence, candidates should read about Ramsar Sites and the Ramsar Convention for UPSC preparation. Read on to get the relevant facts about Ramsar Sites and the list of Ramsar Sites. Ramsar Sites in India - Latest Addition In December 2020, the Tso Kar Wetland Complex was added to the list of Ramsar sites in India. This includes the high-altitude wetland complex of two connected lakes, Startsapuk Tso and Tso Kar, in Ladakh. The following sites have been added as the recognized Ramsar Sites in India: 1. Maharashtra - Lonar Lake 2. Agra (Uttar Pradesh) - Sur Sarovar also called, Keetham Lake 3. Uttarakhand - Asan Barrage 4. Bihar - Kanwar Lake or Kabal Taal Facts about Ramsar Sites & Indian Wetlands The table below provides relevant facts in brief for the use in UPSC Exam: Ramsar Sites in India & Indian Wetlands What are Ramsar Any wetland site which has been listed under the Ramsar Convention that aims to Sites? conserve it and promote sustainable use of its natural resources is called a Ramsar Site. Ramsar Convention is known as the Convention of Wetlands. It was established in 1971 What is the Ramsar by UNESCO and came into force in 1975.