(Hemidactylus Platyurus) Living in Buildings
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Distribution and Habitat of the Invasive Giant Day Gecko Phelsuma Grandis Gray 1870
Phelsuma 22 (2014); 13-28 Distribution and habitat of the invasive giant day gecko Phelsuma grandis Gray 1870 (Sauria: Gekkonidae) in Reunion Island, and conservation implication Mickaël Sanchez1,* and Jean-Michel Probst1,2 1 Nature Océan Indien, 6, Lotissement les Magnolias, Rivière des Roches, 97470 Saint-Benoît, La Réunion, France 2 Nature et Patrimoine, 2, Allée Mangaron, Dos d’Ane, 97419 La Possession, La Réunion, France * Corresponding author; e-mail: [email protected] Abstract. The giant day gecko Phelsuma grandis, endemic to Madagascar, was introduced to Reunion Island (Indian Ocean) in the mid-1990s. No studies have been conducted so far to define its precise distribution and habitat. To fill the knowledge gap about this invasive species, we compiled available data and performed field work during 2007-2014. We detected 13 distinct populations of P. grandis, occurring mainly in the northern part of Reunion Island and on the west coast. This gecko inhabits human disturbed areas (gardens, urban parks, bamboos, orchards, coconuts, and banana plantation) and secondary habitats (shrubby savanna, and secondary dry woodlands, secondary dry and wet thickets). Its distribution strongly suggests that saltatory dispersal (through deliberate and/or accidental transport) and natural colonization are the mechanisms of spreading through Reunion Island. All our data in combination with both P. grandis ecology and native environmental range suggested that this gecko may colonize native forest, and constitutes a potential important threat to the native biodiversity of Reunion Island (arthropods and lizards). Key words. Phelsuma grandis, Reunion Island, distribution, invasive species, conservation. Introduction Day geckos of the genus Phelsuma are distributed in the western Indian Ocean (Austin et al. -

The Trade in Tokay Geckos in South-East Asia
Published by TRAFFIC, Petaling Jaya, Selangor, Malaysia © 2013 TRAFFIC. All rights reserved. All material appearing in this publication is copyrighted and may be reproduced with permission. Any reproduction in full or in part of this publication must credit TRAFFIC as the copyright owner. The views of the authors expressed in this publication do not necessarily reflect those of the TRAFFIC Network, WWF or IUCN. The designations of geographical entities in this publication, and the presentation of the material, do not imply the expression of any opinion whatsoever on the part of TRAFFIC or its supporting organizations concerning the legal status of any country, territory, or area, or its authorities, or concerning the delimitation of its frontiers or boundaries. The TRAFFIC symbol copyright and Registered trademark ownership is held by WWF. TRAFFIC is a strategic alliance of WWF AND IUCN. Layout by Olivier S Caillabet, TRAFFIC Suggested citation: Olivier S. Caillabet (2013). The Trade in Tokay Geckos Gekko gecko in South-East Asia: with a case study on Novel Medicinal Claims in Peninsular Malaysia TRAFFIC, Petaling Jaya, Selangor, Malaysia ISBN 978-983-3393-36-7 Photograph credit Cover: Tokay Gecko in Northern Peninsular Malaysia (C. Gomes/TRAFFIC) The Trade in Tokay Geckos Gekko gecko in South-East Asia: with a case study on Novel Medicinal Claims in Peninsular Malaysia Olivier S. Caillabet © O.S. Caillabet/TRAFFIC A pet shop owner in Northern Peninsular Malaysia showing researchers a Tokay Gecko for sale TABLE OF CONTENTS Acknowledgements -

Sleep-Site Fidelity in Cuban Green
HTTPS://JOURNALS.KU.EDU/REPTILESANDAMPHIBIANSTABLE OF CONTENTS IRCF REPTILES & AMPHIBIANSREPTILES • VOL & AMPHIBIANS15, NO 4 • DEC 2008 • 28(2):189 245–247 • AUG 2021 IRCF REPTILES & AMPHIBIANS CONSERVATION AND NATURAL HISTORY TABLE OF CONTENTS Sleep-siteFEATURE ARTICLES Fidelity in Cuban Green Anoles, . Chasing Bullsnakes (Pituophis catenifer sayi) in Wisconsin: On the Road Anolisto Understanding the Ecology porcatus and Conservation of the Midwest’s Gray Giant Serpent ......................1840 Joshua M. Kapfer 190 . The Shared History of Treeboas (Corallus grenadensis) and Humans on Grenada: A Hypothetical Excursion(Squamata: ............................................................................................................................ Dactyloidae)Robert W. Henderson 198 RESEARCH ARTICLES . The Texas Horned Lizard in Central and Western TexasLuis ....................... F. de Armas Emily Henry, Jason Brewer, Krista Mougey, and Gad Perry 204 . The Knight Anole (Anolis equestris) in Florida .............................................P.O. Box 4327, SanBrian Antonio J. Camposano, de los Baños,Kenneth ArtemisaL. Krysko, Kevin38100, M. CubaEnge, Ellen([email protected]) M. Donlan, and Michael Granatosky 212 CONSERVATION ALERT Photographs by the author. World’s Mammals in Crisis ............................................................................................................................................................. 220 . More Than Mammals ..................................................................................................................................................................... -

Literature Cited in Lizards Natural History Database
Literature Cited in Lizards Natural History database Abdala, C. S., A. S. Quinteros, and R. E. Espinoza. 2008. Two new species of Liolaemus (Iguania: Liolaemidae) from the puna of northwestern Argentina. Herpetologica 64:458-471. Abdala, C. S., D. Baldo, R. A. Juárez, and R. E. Espinoza. 2016. The first parthenogenetic pleurodont Iguanian: a new all-female Liolaemus (Squamata: Liolaemidae) from western Argentina. Copeia 104:487-497. Abdala, C. S., J. C. Acosta, M. R. Cabrera, H. J. Villaviciencio, and J. Marinero. 2009. A new Andean Liolaemus of the L. montanus series (Squamata: Iguania: Liolaemidae) from western Argentina. South American Journal of Herpetology 4:91-102. Abdala, C. S., J. L. Acosta, J. C. Acosta, B. B. Alvarez, F. Arias, L. J. Avila, . S. M. Zalba. 2012. Categorización del estado de conservación de las lagartijas y anfisbenas de la República Argentina. Cuadernos de Herpetologia 26 (Suppl. 1):215-248. Abell, A. J. 1999. Male-female spacing patterns in the lizard, Sceloporus virgatus. Amphibia-Reptilia 20:185-194. Abts, M. L. 1987. Environment and variation in life history traits of the Chuckwalla, Sauromalus obesus. Ecological Monographs 57:215-232. Achaval, F., and A. Olmos. 2003. Anfibios y reptiles del Uruguay. Montevideo, Uruguay: Facultad de Ciencias. Achaval, F., and A. Olmos. 2007. Anfibio y reptiles del Uruguay, 3rd edn. Montevideo, Uruguay: Serie Fauna 1. Ackermann, T. 2006. Schreibers Glatkopfleguan Leiocephalus schreibersii. Munich, Germany: Natur und Tier. Ackley, J. W., P. J. Muelleman, R. E. Carter, R. W. Henderson, and R. Powell. 2009. A rapid assessment of herpetofaunal diversity in variously altered habitats on Dominica. -

Habitat Use by an Endemic and a Non-Native Gecko: Natural Habitat Provides a Last Refuge for the Barbados Leaf-Toed Gecko
Neotropical Biodiversity ISSN: (Print) (Online) Journal homepage: https://www.tandfonline.com/loi/tneo20 Habitat use by an endemic and a non-native gecko: natural habitat provides a last refuge for the Barbados Leaf-Toed gecko Robert J. Williams , Julia A. Horrocks & Angelo P. Pernetta To cite this article: Robert J. Williams , Julia A. Horrocks & Angelo P. Pernetta (2020) Habitat use by an endemic and a non-native gecko: natural habitat provides a last refuge for the Barbados Leaf-Toed gecko, Neotropical Biodiversity, 6:1, 127-137, DOI: 10.1080/23766808.2020.1804750 To link to this article: https://doi.org/10.1080/23766808.2020.1804750 © 2020 The Author(s). Published by Informa UK Limited, trading as Taylor & Francis Group. Published online: 17 Aug 2020. Submit your article to this journal Article views: 35 View related articles View Crossmark data Full Terms & Conditions of access and use can be found at https://www.tandfonline.com/action/journalInformation?journalCode=tneo20 NEOTROPICAL BIODIVERSITY 2020, VOL. 6, NO. 1, 127–137 https://doi.org/10.1080/23766808.2020.1804750 Habitat use by an endemic and a non-native gecko: natural habitat provides a last refuge for the Barbados Leaf-Toed gecko Robert J. Williams a,b, Julia A. Horrocksc and Angelo P. Pernettab aSchool of Biology, University of Leeds, Leeds, UK; bConservation and Zoonosis Research and Enterprise Group, School of Pharmacy and Biomolecular Sciences, University of Brighton, Brighton, UK; cDepartment of Biological and Chemical Sciences, University of the West Indies, Barbados, West Indies ABSTRACT ARTICLE HISTORY Island endemic reptiles face many threats that potentially have a negative effect on their Received 8 January 2020 conservation status, such as habitat loss, interactions with introduced predators and compe Accepted 9 June 2020 titors, and stochastic environmental events. -

Introduced Amphibians and Reptiles in the Cuban Archipelago
Herpetological Conservation and Biology 10(3):985–1012. Submitted: 3 December 2014; Accepted: 14 October 2015; Published: 16 December 2015. INTRODUCED AMPHIBIANS AND REPTILES IN THE CUBAN ARCHIPELAGO 1,5 2 3 RAFAEL BORROTO-PÁEZ , ROBERTO ALONSO BOSCH , BORIS A. FABRES , AND OSMANY 4 ALVAREZ GARCÍA 1Sociedad Cubana de Zoología, Carretera de Varona km 3.5, Boyeros, La Habana, Cuba 2Museo de Historia Natural ”Felipe Poey.” Facultad de Biología, Universidad de La Habana, La Habana, Cuba 3Environmental Protection in the Caribbean (EPIC), Green Cove Springs, Florida, USA 4Centro de Investigaciones de Mejoramiento Animal de la Ganadería Tropical, MINAGRI, Cotorro, La Habana, Cuba 5Corresponding author, email: [email protected] Abstract.—The number of introductions and resulting established populations of amphibians and reptiles in Caribbean islands is alarming. Through an extensive review of information on Cuban herpetofauna, including protected area management plans, we present the first comprehensive inventory of introduced amphibians and reptiles in the Cuban archipelago. We classify species as Invasive, Established Non-invasive, Not Established, and Transported. We document the arrival of 26 species, five amphibians and 21 reptiles, in more than 35 different introduction events. Of the 26 species, we identify 11 species (42.3%), one amphibian and 10 reptiles, as established, with nine of them being invasive: Lithobates catesbeianus, Caiman crocodilus, Hemidactylus mabouia, H. angulatus, H. frenatus, Gonatodes albogularis, Sphaerodactylus argus, Gymnophthalmus underwoodi, and Indotyphlops braminus. We present the introduced range of each of the 26 species in the Cuban archipelago as well as the other Caribbean islands and document historical records, the population sources, dispersal pathways, introduction events, current status of distribution, and impacts. -

Phyllomedusa 19-2 INGLÊS.Indd
Phyllomedusa 19(1):273–277, 2020 © 2020 Universidade de São Paulo - ESALQ ISSN 1519-1397 (print) / ISSN 2316-9079 (online) doi: http://dx.doi.org/10.11606/issn.2316-9079.v19i1p273-277 SHORT COMMUNICATION Envenomation records of Hemidactylus mabouia (Squamata: Gekkonidae) by Ctenus rectipes (Araneae: Ctenidae) in an urban area of northeastern Brazil Gabriela Quintela Cavalcante Correia,1 Marcos Jorge Matias Dubeux,2,3,4 Filipe Augusto Cavalcanti do Nascimento,3,4 Selma Torquato,3 and Tamí Mott3,4 1 Setor de Entomologia, Museu de História Natural, Universidade Federal de Alagoas. Av. Amazonas s/n, Prado, 57010-060, Maceió, AL, Brazil. 2 Programa de Pós-Graduação em Biologia Animal, Departamento de Zoologia, Centro de Biociências, Universidade Federal de Pernambuco. Av. Professor Moraes Rego s/n. 50670-901, Recife, PE, Brazil. E-mail: [email protected]. 3 Setor de Herpetologia, Museu de História Natural, Universidade Federal de Alagoas. Av. Amazonas s/n, Prado, 57010-060, Maceió, AL, Brazil. 4 Laboratório de Biologia Integrativa, Setor de Biodiversidade, Universidade Federal de Alagoas. Av. Lourival Melo Mota s/n, Tabuleiro, 57072-970, Maceió, AL, Brazil. Keywords: Exotic species, lizard, envenomation, predation, ctenid spider. Palavras-chave: aranha, espécie exótica, lagarto, envenenamento, predação. Hemidactylus mabouia (Moreau-De-Jonnès, coastal islands (Rocha et al. 2011). Hemidactylus 1818), popularly known as Tropical House mabouia is a generalist and opportunist regarding Gecko, is a nocturnal lizard of African origin its diet, and is known to feed on diverse groups that is well adapted to human dwellings (Jesus et of arthropods, including spiders (Zamprogno and al. 2001, Short and Petren 2012). -
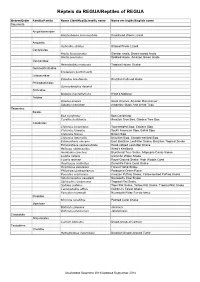
REGUA Reptile List
Répteis da REGUA/Reptiles of REGUA Ordem/Order Família/Family Nome Científico/Scientific name Nome em Inglês/English name Squamata Amphisbaenidae Amphisbaena microcephala Smallhead Worm Lizard Anguidae Ophiodes striatus Striped Worm Lizard Dactyloidae Anolis fuscoauratus Slender anole, Brown-eared Anole Anolis punctatus Spotted Anole, Amazon Green Anole Gekkonidae Hemidactylus mabouia Tropical House Gecko Gymnophtalmidae Ecpleopus gaudichaudii Leiosauridae Enyalius brasiliensis Brazilian Fathead Anole Phyllodactylidae Gymnodactylus darwinii Scincidae Mabuya macrorhyncha Hoge's Mabuya Teiidae Ameiva ameiva Giant Ameiva, Amazon Racerunner Salvator merianae Argentine Black And White Tegu Serpentes Boidae Boa constrictor Boa Constrictor Corallus hortulanus Amazon Tree Boa, Garden Tree Boa Colubridae Chironius bicarinatus Two-headed Sipo, Eastern Sipo Chironius foveatus South American Sipo, Bahia Sipo Chironius fuscus Brown Sipo Chironius laevicollis Brazilian Sipo, Smooth-necked Sipo Echinanthera amoena East Brazilian Leaf-litter Snake, Brazilian Tropical Snake Echinanthera cephalostriata Head-striped Leaf-litter Snake Helicops carinicaudus Wied's Keelback Imantodes cenchoa Blunthead Tree Snake, Mapepire Corde Violon Liophis miliaris Common Water Snake Liophis reginae Royal Ground Snake, High Woods Coral Oxyrhopus clathratus Duméril's False Coral Snake Oxyrhopus petolarius Forest Flame Snake Philodryas patagoniensis Patagonia Green Racer Pseustes sulphureus Amazon Puffing Snake, Yellow-bellied Puffing Snake Sibynomorphus neuwiedi Neuwied's Tree Snake Siphlophis compressus Tropical Flat Snake Spilotes pullatus Tiger Rat Snake, Yellow Rat Snake, Tropical Rat Snake Taeniophallus affinis Günther's Forest Snake Xenodon neuwiedii Neuwied's False Fer-de-lance Elapidae Micrurus corallinus Painted Coral Snake Viperidae Bothrops jararaca Jararaca Bothrops jararacussu Jararacussu Crocodylia Alligatoridae Caiman latirostris Broad-snouted Caiman Testudines Chelidae Hydromedusa maximiliani Brazilian Snake-necked Turtle Atualizados Setembro 2014/Updated September 2014. -

Opportunistic Feeding by House-Dwelling Geckos: Does This Make Them More Successful Invaders?
The Herpetological Bulletin 149, 2019: 38-40 SHORT COMMUNICATION https://doi.org/10.33256/hb149.3840 Opportunistic feeding by house-dwelling geckos: does this make them more successful invaders? ROBBIE WETERINGS1* & PREEYAPORN WETERINGS1 1 Cat Drop Foundation, Drachten, Netherlands *Corresponding author e-mail: [email protected] Abstract - Various species of ‘house’ gecko are found in and around buildings, where they can be observed feeding opportunistically on the insects attracted to artificial lights. Most of the species are considered strict insectivores. Nevertheless, there have been several recently published observations of ‘house’ geckos feeding on non-insect food. In order to assess how common this behaviour is among geckos worldwide, we offered an online questionnaire to ecologists and herpetologists. Of the 74 observations received, most reported Hemidactylus frenatus, H. platyurus and Gehyra mutilata feeding on rice, bread, fruits, vegetables, dog food or chocolate cream, taken from tables, plates, and garbage bins. This opportunistic feeding behaviour is much more common than previously thought and is perpetrated by species considered to be highly invasive, possibly contributing to their success as invaders. INTRODUCTION 2. What did the gecko consume? a. Insects or other invertebrates everal gecko species (e.g. Hemidactylus frenatus and b. Fruit or vegetables SGehyra mutilata) are often found in and around houses. c. Rice These, so-called ‘house’ geckos, are very well adapted to d. Bread urban life and are often observed feeding opportunistically e. Eggs on insects attracted to artificial lights at night (Tkaczenko f. Unsure et al., 2014). This provides them an easily accessible food g. Other... source in locations generally lacking predators. -

Natural History of the Gecko Hemidactylus Prashadi: Demography, Spatial Partitioning, Diet, and Reproduction in a Human-Altered Habitat
Herpetological Conservation and Biology 16(2):325–336. Submitted: 11 April 2021; Accepted: 6 July 2021; Published: 31 August 2021. NATURAL HISTORY OF THE GECKO HEMIDACTYLUS PRASHADI: DEMOGRAPHY, SPATIAL PARTITIONING, DIET, AND REPRODUCTION IN A HUMAN-ALTERED HABITAT VIVEK PHILIP CYRIAC1,3,4 AND P. K. UMESH2 1Centre for Wildlife Studies, College of Veterinary and Animal Science, Pookode, Wayanad - 673576, Kerala, India 2Pavuknandy House, Moolad Post Office, Balussery, Kozhikode - 673614, Kerala, India 3Current Address: Centre for Ecological Sciences, Indian Institute of Science, Bangalore - 560012, Karnataka, India 4Corresponding author, email: [email protected] Abstract.—Human-induced alterations have had a profound impact on the environment affecting several species. Many lizards, however, especially members of the gecko genus Hemidactylus, are cosmopolitan and are found living on buildings in urban areas. Nevertheless, how some reptiles colonize and thrive in human-altered habitats remain relatively less explored, partly due to the lack of adequate natural history on different species. Here, we study the natural history of Prashad’s Gecko (Hemidactylus prashadi), a poorly studied, large-bodied gecko, which is believed to have recently colonized houses in the study region. We report new populations of this species extending the range further south to Kerala, India. We also studied the demographic structure, spatial partitioning, diet, and reproduction of the lizard in a residential building in Kozhikode district, Kerala. We found the population in the building was dominated by adult females and juveniles, while adult males and sub-adults were few. Perch height and non-lethal injuries of individuals on the building suggest intense intraspecific competition and spatial partitioning between juveniles and adults. -

Broken Screens: the Regulation of Live Animal Imports in the United States
Broken Screen S The Regulation of Live Animal Imports in the United States DEFENDERS OF WILDLIFE Defenders of Wildlife is a national, nonprofit membership organization dedicated to the protection of all native wild animals and plants in their natural communities. PROJECT CONTRIBUTORS The Consortium for Conservation Medicine (CCM) is a collaborative institution linking Johns Hopkins Bloomberg School of Public Health, Tufts University School of Veterinary Medicine Center for Conservation Medicine, The University of Pittsburgh Graduate School of Public Health, the University of Wisconsin-Madison Nelson Institute for Environmental Studies, the U.S. Geological Society National Wildlife Health Center and the Wildlife Trust. CCM strives to understand the links among human changes to the environment, the health of all species including humans, and the conservation of biodiversity. www.conservationmedicine.org The Invasive Species Specialist Group (ISSG) is part of the Species Survival Commission of The World Conservation Union (IUCN). The ISSG consist of about 150 scientific and policy experts on invasive species from more than 40 countries. The ISSG aims to reduce threats to natural ecosystems and the native species they contain by increasing awareness of invasive alien species, and of ways to prevent, control or eradicate them. www.issg.org ACKNOWLEDGEMENTS Defenders of Wildlife Principal Author: Peter T. Jenkins Co-authors: Kristen Genovese, Heidi Ruffler Additional assistance: Carroll Muffett, Stas Burgiel, Kelly Malsch, Timm Kroeger, Mark Cheater, Robert Irvin and Gabriela Chavarria Researcher: David Tucker Editor: Kate Davies Art Director: Jen Lee Consortium for Conservation Medicine Principal Contributor: Katherine F. Smith Additional assistance: Peter Daszak and Lisa Schloegel IUCN Invasive Species Specialist Group Principal Contributor: Michael Browne Additional assistance: Shyama Pagad, UniServices Ltd. -

Uncovered Genetic Diversity in Hemidactylus Mabouia (Reptilia: Gekkonidae) from Madeira Island Reveals Uncertain Sources of Introduction
bioRxiv preprint doi: https://doi.org/10.1101/2021.03.02.433159; this version posted March 2, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. Short Note Uncovered genetic diversity in Hemidactylus mabouia (Reptilia: Gekkonidae) from Madeira Island reveals uncertain sources of introduction Catarina Rato1*, Beatriz Martins1,2, Ricardo Rocha1,3†, Iolanda Silva-Rocha1† 1 CIBIO, Research Centre in Biodiversity and Genetic Resources, InBIO, Universidade do Porto, Campus de Vairão, Rua Padre Armando Quintas nº7, Vairão 4485 - 661, Vila do Conde, Portugal. 2 Departamento de Biologia, Faculdade de Ciências da Universi dade do Porto, R. Campo Alegre, s/n, 4169 - 007, Porto, Portugal. 3 CIBIO- InBIO, Research Center in Biodiversity and Genetic Resources, Institute of Agronomy, University of Lisbon, Lisbon, Portugal † These authors have contributed equally to this work * Corresponding author: [email protected] Total number of words in the whole manuscript: 3,274 Total number of words in the abstract: 151 1 bioRxiv preprint doi: https://doi.org/10.1101/2021.03.02.433159; this version posted March 2, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. 36 Abstract 37 Hemidactylus mabouia is one of the most widely distributed species within its genus.