Identification of Small Molecule Modulators of Diguanylate Cyclase
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Addressing 360O of Biochemical Imbalances to Restore Balance and Relieve Symptoms
Addressing 360o of biochemical imbalances to restore balance and relieve symptoms. Product Guide Effective June 2015 Product availability subject to change without notice. View the most current catalog electronically at www.neuroscienceinc.com/productcatalog Dr. Gottfried Kellermann and Mieke Kellermann Ushering in a New Age of Personalized Care As NeuroScience, Inc. enters its 15th year in business, we reflect on the driving force behind our services- providing you, the practitioner, with tools that allow you to more efficiently and effectively care for your patients. Together with our laboratory partner, Pharmasan Labs, Inc. we pioneered the Assess and Address™ approach for personalized patient care. New and Exciting Direction We recognize the evolving changes in patient care and patient expectation from their healthcare practitioners. More than ever, patients are taking control of their health and demanding care that allows them to get better faster and enjoy the healthy lifestyle they deserve. NeuroScience, along with Pharmasan Labs, has taken the lead in focusing on a model that not only identifies the imbalances behind many symptoms but how the network of those imbalances indicates deeper issues. This network approach points you in the direction of the root cause of patient symptoms, which when resolved can lead to improved, long-term health outcomes. Never satisfied with the status quo, we will continue to innovate to provide you with unparalleled support toward this end. A Provider of Personalized, Clinical Solutions…this is WHO WE ARE. Together, NeuroScience and Pharmasan are committed to providing you with the clinical assessments and tools you need to thrive in this rapidly changing healthcare market while better serving your patients. -

Integrated Approach for Identifying the Molecular, Cellular, and Host Responses to Chemical Insults
Integrated Approach for Identifying the Molecular, Cellular, and Host Responses to Chemical Insults Audrey E. Fischer, Emily P. English, Julia B. Patrone, Kathlyn Santos, Jody B. G. Proescher, Rachel S. Quizon, Kelly A. Van Houten, Robert S. Pilato, Eric J. Van Gieson, and Lucy M. Carruth e performed a pilot study to characterize the molecular, cellular, and whole-organism response to nonlethal chemical agent exposure in the central nervous system. Multiple methodologies were applied to measure in vitro enzyme inhibition, neuronal cell pathway signaling, and in vivo zebrafish neural development in response to challenge with two different classes of chemical compounds. While all compounds tested exhibited expected enzyme inhibitory activity against acetylcholinesterase (AChE), a well-characterized target of chemical agents, distinct differences between chemical exposures were detected in cellular toxicity and pathway target responses and were tested in a zebrafish model. Some of these differences have not been detected using conventional chemical toxicity screening methods. Taken together, the data demonstrate the potential value of an integrated, multimethodological approach for improved target and pathway identification for subsequent diagnostic and therapeutic biomarker development. INTRODUCTION To build capability and leverage new and growing cell models to complete living organisms. Regardless of biology and chemistry expertise at APL, a collabora- the model selected, challenges exist in sample collection, tive, cross-departmental effort was established through a dose determination, and biases inherent in each assay/ series of related independent research and development technology. Therefore, multiple experimental methodol- (IR&D) projects. The focus of this effort was on mitiga- ogies brought to bear on a particular biological question tion of chemical and biological threat agents. -

United States Patent (19) 11 4,134,792 Boguslaski Et Al
United States Patent (19) 11 4,134,792 Boguslaski et al. 45) Jan. 16, 1979 54 SPECIFIC BINDING ASSAY WITH AN Immunoassays', Conference on Non-isotopic Immuno ENZYMEMODULATOR ASALABELNG assays, (1976). SUBSTANCE Primary Examiner-Alvin E. Tanenholtz 75) Inventors: Robert C. Boguslaski; Robert J. Assistant Examiner-C. A. Fan Carrico, both of Elkhart, Ind; James Attorney, Agent, or Firm-Andrew L. Klawitter E. Christner, Ann Arbor, Mich. 57 ABSTRACT A specific binding assay method employing, as a label 73) Assignee: Miles Laboratories, Inc., Elkhart, ing substance, a reversibly binding enzyme modulator Ind. for the detection of a ligand in a liquid medium. The 21 Appl. No.: 748,005 method follows conventional specific binding assay techniques of either the homogeneous or heterogeneous (22 Filed: Dec. 6, 1976 type wherein the liquid medium to be assayed is com bined with reagent means that includes a labeled conju 51) int. C.?...................... G01N 33/00; G01N 31/14 gate to form a binding reaction system having a bound 52 U.S. C. .............................. 195/99; 195/103.5A; species and a free-species of the conjugate. The amount 424/12 of conjugate resulting in the bound-species or the free 58) Field of Search .......................... 195/103.5 A, 99; species is a function of the amount of ligand present in 424/12 the liquid medium assayed. In the present invention, the labeled conjugate comprises a reversibly binding en 56 References Cited zyme modulator covalently linked to a binding compo U.S. PATENT DOCUMENTS nent of the binding reaction system. The distribution of 3,817,837 6/1974 Rubenstein et al. -

Genome Biology
Genome Biology This Provisional PDF corresponds to the article as it appeared upon acceptance. Copyedited and fully formatted PDF and full text (HTML) versions will be made available soon. The draft genome and transcriptome of Cannabis sativa Genome Biology 2011, 12:R102 doi:10.1186/gb-2011-12-10-r102 Harm van Bakel ([email protected]) Jake M Stout ([email protected]) Atina G Cote ([email protected]) Carling M Tallon ([email protected]) Andrew G Sharpe ([email protected]) Timothy R Hughes ([email protected]) Jonathan E Page ([email protected]) ISSN 1465-6906 Article type Research Submission date 11 September 2011 Acceptance date 20 October 2011 Publication date 20 October 2011 Article URL http://genomebiology.com/2011/12/10/R102 This peer-reviewed article was published immediately upon acceptance. It can be downloaded, printed and distributed freely for any purposes (see copyright notice below). Articles in Genome Biology are listed in PubMed and archived at PubMed Central. For information about publishing your research in Genome Biology go to http://genomebiology.com/authors/instructions/ © 2011 van Bakel et al. ; licensee BioMed Central Ltd. This is an open access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. The draft genome and transcriptome of Cannabis sativa Harm van Bakel1, Jake M Stout2,4, Atina G Cote1, Carling M Tallon2, Andrew G Sharpe2, Timothy R Hughes1,3* and Jonathan E Page2,4* 1Banting and Best Department of Medical Research and Terrence Donnelly Centre for Cellular and Biomolecular Research, University of Toronto, 160 College St. -

The Thermo-Sensitive Gene Expression Signatures of Spermatogenesis Santosh K
Yadav et al. Reproductive Biology and Endocrinology (2018) 16:56 https://doi.org/10.1186/s12958-018-0372-8 RESEARCH Open Access The thermo-sensitive gene expression signatures of spermatogenesis Santosh K. Yadav1†, Aastha Pandey1†, Lokesh Kumar1, Archana Devi1,2, Bhavana Kushwaha1,2, Rahul Vishvkarma1, Jagdamba P. Maikhuri1, Singh Rajender1,2 and Gopal Gupta1,2* Abstract Background: Spermatogenesis in most mammals (including human and rat) occurs at ~ 3 °C lower than body temperature in a scrotum and fails rapidly at 37 °C inside the abdomen. The present study investigates the heat-sensitive transcriptome and miRNAs in the most vulnerable germ cells (spermatocytes and round spermatids) that are primarily targeted at elevated temperature in a bid to identify novel targets for contraception and/or infertility treatment. Methods: Testes of adult male rats subjected to surgical cryptorchidism were obtained at 0, 24, 72 and 120 h post-surgery, followed by isolation of primary spermatocytes and round spermatids and purification to > 90% purity using a combination of trypsin digestion, centrifugal elutriation and density gradient centrifugation techniques. RNA isolated from these cells was sequenced by massive parallel sequencing technique to identify the most-heat sensitive mRNAs and miRNAs. Results: Heat stress altered the expression of a large number of genes by ≥2.0 fold, out of which 594 genes (286↑; 308↓) showed alterations in spermatocytes and 154 genes (105↑;49↓) showed alterations in spermatids throughout the duration of experiment. 62 heat-sensitive genes were common to both cell types. Similarly, 66 and 60 heat-sensitive miRNAs in spermatocytes and spermatids, respectively, were affected by ≥1.5 fold, out of which 6 were common to both the cell types. -

Introduction
Introduction ECNP is an independent, non-governmental, scientific association dedicated to the science and treatment of disorders of the brain. Founded in 1987, its goal is to bring together scientists and clinicians to facilitate information-sharing and spur new discoveries. The objective of ECNP is to serve the public good by stimulating high-quality experimental and clinical research and education in applied and translational neuroscience. It seeks to do this by: Co-ordinating and promoting scientific activities and consistently high-quality standards between countries in Europe. Bringing together all those involved in or interested in the scientific study of applied and translational neuroscience by arranging scientific meetings, seminars, and study groups. Providing guidance and information to the public on matters relevant to the field. Providing a format for the co-ordination and for development of common standards in Europe. To fulfil this aim ECNP organises, amongst others, yearly the ECNP Congress that comprises of 6 plenary lectures, 28 symposia and 7 educational update sessions. The annual meeting attracts more than 6,000 participants and is considered to be the largest event in neuropsychopharmacology in Europe. ECNP organises seminars, as the one you have been invited to participate, in areas of Europe where there are less opportunities for psychiatrists to participate in international meetings. Interaction is the keyword at these meetings and they have proved very successful both for the participants and for the experts. During the seminar we discuss clinical and research issues that the local organisers feel that are needed to be covered and using these topics as a model for teaching how to ask a research question and how to plan an effective study. -

Dietary Phytoestrogens: Potential Selective Estrogen Jean-Philippe Basly1 Marie-Chantal Canivenc Lavier2 Enzyme Modulators? Review
Dietary Phytoestrogens: Potential Selective Estrogen Jean-Philippe Basly1 Marie-Chantal Canivenc Lavier2 Enzyme Modulators? Review Abstract phytoestrogens, this contribution covers the estrogen pathway inhibition effects of these compounds and special attention will Between one-third to one-half of all breast cancers are steroid be given to isoflavonoids, flavonoids and lignans. Furthermore, sensitive. Steroid-pathway enzymes (sulfatase, 17b-hydroxyster- the research on structurally-related compounds as therapeutic oid dehydrogenases, aromatase and sulfotransferases) are thus agents will be discussed briefly. prime candidates for therapeutic approaches based on the con- trol of intacrine activity. Some phytoestrogens, ubiquitous in Key words our diet, are inhibitors of these enzymes. Such a therapeutic po- Phytoestrogens ´ aromatase ´ hydroxysteroid dehydrogenases ´ tential has stimulated research and progress has been achieved sulfatase ´ sulfotransferases ´ endocrine disrupters during the last years. Complementary to previous reviews on Introduction ªaromatase pathwayº which respectively transforms androste- nedione (AD) to estrone (E1) and testosterone (T) to estradiol Over the past 15 years, there has been a tremendous increase in (E2) and (ii) the ªsulfatase pathwayº which converts estrone sul- 287 the number of papers published on the biological activities of fate (E1S) into estrone (E1); estrone is then transformed into es- phytoestrogens. The estrogen receptor binding is the best-docu- tradiol (E2)by17b-hydroxysteroid dehydrogenase type 1. By ana- mented biological action of phytoestrogens and, as estrogen ago- logy with a selected estrogen receptor modulator (SERM), the nists and antagonists, they can be classified as selective estrogen concept of a selective estrogen enzyme modulator (SEEM) as a receptor modulators (SERMs) [1]. These compounds also have a therapeutic agent has recently emerged [3], [4]. -

An Overview of FDA-Approved Biologics Medicines
Drug Discovery Today Volume 20, Number 4 April 2015 PERSPECTIVE feature An overview of FDA-approved biologics medicines Michael S. Kinch, [email protected], [email protected] Recombinant DNA technologies revolutionized medicine. Herein, the approvals and mechanistic basis of biologics-based medicines are analyzed. The overall and relative rate of FDA approvals for recombinant proteins grew from the 1980s through the first half-decade of the new millennium. Over time, the number of biologics gaining approval for an orphan indication has climbed to more than 50% in the current decade. The field has been dynamic in terms of the types of biologics, indications targeted and the mechanistic basis of drug activity. Despite impressive increases in recombinant-protein-based medicine, the rate of new biologics approvals could have leveled out. PERSPECTIVE Current analysis concerns that science would outpace society’s established pharmaceutical company (Eli Lilly) Recombinant DNA technology fundamentally ability to control new technology safely. and gained FDA approval for the first recombi- 1 Features changed the way medicines are discovered and Research of more-controversial aspects of nant DNA product (Humulin ; 1982) [5,6]. This developed. In a remarkable convergence of recombinant DNA research was voluntarily sus- aggressive timeline set a standard that continues timing and talent, Stanford University was the pended shortly after the publication of the first to be associated with the biotechnology industry epicenter of a revolution in the -
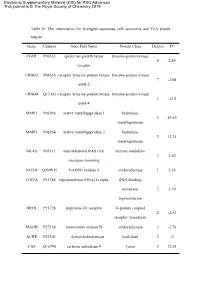
Table S1 the Information for Laryngeal Squamous Cell Carcinoma and TFA Shared Targets
Electronic Supplementary Material (ESI) for RSC Advances. This journal is © The Royal Society of Chemistry 2019 Table S1 The information for laryngeal squamous cell carcinoma and TFA shared targets. Gene Uniprot Gene Full Name Protein Class Degree FC EGFR P00533 epidermal growth factor tyrosine-protein kinase 9 2.49 receptor ERBB2 P04626 receptor tyrosine-protein kinase tyrosine-protein kinase 7 -2.08 erbB-2 ERBB4 Q15303 receptor tyrosine-protein kinase tyrosine-protein kinase 3 -13.5 erbB-4 MMP1 P03956 matrix metallopeptidase 1 hydrolase; 3 49.49 metalloprotease MMP3 P08254 matrix metallopeptidase 3 hydrolase; 2 13.34 metalloprotease NRAS P01111 neuroblastoma RAS viral enzyme modulator 3 2.50 oncogene homolog NOX4 Q9NPH5 NADPH oxidase 4 oxidoreductase 1 2.34 TOP2A P11388 topoisomerase (DNA) II alpha DNA-binding; isomerase; 2 3.70 topoisomerase DRD1 P21728 dopamine D1 receptor G-protein coupled 2 -2.53 receptor; transducer MAOB P27338 monoamine oxidase B oxidoreductase 2 -2.70 ACHE P22303 Acetylcholinesterase hydrolase 2 -2 CA9 Q16790 carbonic anhydrase 9 lyase 2 12.64 ALDH1A1 P00352 retinal dehydrogenase 1 oxidoreductase 3 -2.50 ALDH1A2 O94788 retinal dehydrogenase 2 oxidoreductase 1 -2.50 ALOX12B O75342 epidermis-type lipoxygenase 12 oxidoreductase 1 2.11 ALOX15 P16050 arachidonate 15-lipoxygenase oxidoreductase 1 -2.61 DCT P40126 dopachrome tautomerase isomerase 0 -4.54 CYP1B1 Q16678 cytochrome P450 1B1 oxidoreductase 0 -2.46 DAPK1 P53355 death associated protein kinase calmodulin-binding; 1 kinase; serine/threonine- 0 2 protein kinase FC: Fold change, the positive value indicates that the cancer is up-regulated compared with the adjacent cancer, and the negative value indicates that the cancer is down- regulated. -

United States Patent (19) 11 Patent Number: 5,919,641 Cromer Et Al
USOO59 19641A United States Patent (19) 11 Patent Number: 5,919,641 Cromer et al. (45) Date of Patent: *Jul. 6, 1999 54) HOMOGENEOUS IMMUNOASSAYS USING FOREIGN PATENT DOCUMENTS ENZYME INHIBITORS 0 154276 A3 9/1985 European Pat. Off.. 0 245981 A3 11/1987 European Pat. Off.. 75 Inventors: Remy Cromer, San Jose, Rohan O 271 731 A3 6/1988 European Pat. Off.. Peries, Mountain View, Dariush 0 272 691 A2 6/1988 European Pat. Off.. Davalian, San Jose; Carl N. Skold, 1595 101 8/1981 United Kingdom Mountain View; Edwin F. Ullman, Atherton; Kesavan Radika, Mountain OTHER PUBLICATIONS View, all of Calif. Ngo, et al., FEBS Letters Jul. 1980, vol. 116(2), 285–288. Skold, et al., Journal of Immunology May 1987, vol. 138, 73 Assignee: Dade Behring Marburg GmbH, 3408-3414. Marburg, Germany Wei, et al., Clinical Chemistry 1977, vol. 23(8), 1386-1388. * Notice: This patent is subject to a terminal dis- Miwake,Es et al.al., Agric. BloBiol. Chem.efit 1988 , VO 1. 52(7(7), claimer. Ashihara, et al., Journal of Clinical Laboratory Analysis 1987, vol. 1, 77-79. 21 Appl. No.: 08/451,326 Primary Examiner. Sheela Huff 22 Filed: May 26, 1995 Attorney, Agent, or Firm- Theodore J Leitereg, Lois K RuSZala Related U.S. Application Data 57 ABSTRACT 62 Division of application No. 07/747,082, Aug. 19, 1991. A method for determining the presence of an analyte which (51) Int. Cl." ..................................................... G01N 33/53 is a specific binding pair member in a Sample Suspected of 52) ... 435/7.71; 435/7.1 containing the analyte is described. -

Drug Repositioning for Enzyme Modulator Based on Human Metabolite-Likeness Yoon Hyeok Lee, Hojae Choi, Seongyong Park, Boah Lee and Gwan-Su Yi*
The Author(s) BMC Bioinformatics 2017, 18(Suppl 7):226 DOI 10.1186/s12859-017-1637-5 RESEARCH Open Access Drug repositioning for enzyme modulator based on human metabolite-likeness Yoon Hyeok Lee, Hojae Choi, Seongyong Park, Boah Lee and Gwan-Su Yi* From DTMBIO 2016: The Tenth International Workshop on Data and Text Mining in Biomedical Informatics Indianapolis, IN, USA. 24-28 October 2016 Abstract Background: Recently, the metabolite-likeness of the drug space has emerged and has opened a new possibility for exploring human metabolite-like candidates in drug discovery. However, the applicability of metabolite-likeness in drug discovery has been largely unexplored. Moreover, there are no reports on its applications for the repositioning of drugs to possible enzyme modulators, although enzyme-drug relations could be directly inferred from the similarity relationships between enzyme’s metabolites and drugs. Methods: We constructed a drug-metabolite structural similarity matrix, which contains 1,861 FDA-approved drugs and 1,110 human intermediary metabolites scored with the Tanimoto similarity. To verify the metabolite-likeness measure for drug repositioning, we analyzed 17 known antimetabolite drugs that resemble the innate metabolites of their eleven target enzymes as the gold standard positives. Highly scored drugs were selected as possible modulators of enzymes for their corresponding metabolites. Then, we assessed the performance of metabolite-likeness with a receiver operating characteristic analysis and compared it with other drug-target prediction methods. We set the similarity threshold for drug repositioning candidates of new enzyme modulators based on maximization of the Youden’s index. We also carried out literature surveys for supporting the drug repositioning results based on the metabolite-likeness. -

Identification and Characterization of a Cardiomyopathy Syndrome
Identification and Characterization of a Cardiomyopathy Syndrome Resulting from Loss of the Melanocortin 4 Receptor By Michael Litt Dissertation Submitted to the Faculty of the Graduate School of Vanderbilt University In partial fulfillment of the requirements for the degree of DOCTOR OF PHILOSOPHY in Molecular Biology and Biophysics August 31, 2017 Nashville, Tennessee Approved: David Wasserman Ph.D. Roger Cone Ph.D. Owen McGuinness Ph.D. Danny Winder Ph.D. David Weaver Ph.D. To Papa Jay and Papa Duck. ii ACKNOWLEDGEMENTS Any perception of my success in graduate school is a direct reflection of the tremendous guidance and support that I have received. First and foremost, I would like to thank Roger Cone for his tireless mentorship and unwavering support. It takes an extremely special mentor to train a graduate student working on an unfunded side project. His ‘sink or swim’ style has given me a confidence I will draw on for the rest of my career. For that, I am forever indebted. I will always be grateful to the guidance of Dr. Javid Moslehi. His ability to provide honest and critical feedback has been sine qua non. His willingness to find the time to help me with my project while simultaneously starting a lab and clinical department are a true testament to his dedication as a mentor. I would like to extend the deepest of thanks to the members of my thesis committee: Dave Wasserman, Owen McGuinness, Danny Winder and Dave Weaver. Their astute and timely insights into my project have provided a sense of clarity to both my science and professional development.