Considerations of HLA, Renal Failure, Valproic Acid Use, and Current Treatment Guidelines in Clozapine-Induced Agranulocytosis
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

PROVERA Medroxyprogesterone Acetate Tablets 2.5Mg, 5Mg, 10Mg, 100 Mg, 200 Mg Tablets
PROVERA Medroxyprogesterone acetate tablets 2.5mg, 5mg, 10mg, 100 mg, 200 mg tablets What is in this leaflet establish a regular menstrual breast cancer or breast lumps cycle not diagnosed by your doctor This leaflet answers some common bleeding or discharge from questions about PROVERA. It does certain types of cancer including your nipples not contain all the available cancer of the breast, kidney and information. It does not take the endometrium (the lining of the miscarriage place of talking to your doctor or womb). pharmacist. cancer of the womb or ovary PROVERA, in combination with an uncontrolled high blood estrogen containing medicine, is All medicines have risks and pressure. benefits. Your doctor has weighed used to relieve symptoms of the risks of you taking PROVERA menopause in women with an intact Do not take PROVERA if you are against the benefits it is expected to uterus. This is called hormone pregnant or intend to become have for you. replacement therapy (HRT). pregnant. PROVERA is used to protect the If you have any concerns about lining of the uterus while the PROVERA may affect your taking this medicine, ask your estrogens relieve the symptoms of developing baby if you take it doctor or pharmacist. Keep this menopause. PROVERA is not during pregnancy. leaflet with your medicine. suitable as a HRT treatment in women who have undergone a Do not take PROVERA if the You may need to read it again. hysterectomy. packaging is torn or shows signs of tampering. Do not take Your doctor may have prescribed PROVERA after the expiry date What PROVERA is PROVERA for another purpose. -

Liquid Spray Formulations for Buccal Delivery of Cannabinoids
(19) TZZ_¥__T (11) EP 1 361 864 B1 (12) EUROPEAN PATENT SPECIFICATION (45) Date of publication and mention (51) Int Cl.: of the grant of the patent: A61K 9/08 (2006.01) A61K 9/107 (2006.01) 04.12.2013 Bulletin 2013/49 A61K 36/185 (2006.01) (21) Application number: 02712063.3 (86) International application number: PCT/GB2002/000620 (22) Date of filing: 14.02.2002 (87) International publication number: WO 2002/064109 (22.08.2002 Gazette 2002/34) (54) LIQUID SPRAY FORMULATIONS FOR BUCCAL DELIVERY OF CANNABINOIDS FLÜSSIGE SPRAY FORMULIERUNGEN ZUR BUCCALEN VERABREICHUNG VON CANNABINOIDEN PREPARATIONS DE SPRAY LIQUIDE POUR L’ADMINISTRATION BUCCALE DE CANNABINOIDES (84) Designated Contracting States: (74) Representative: Schiller, Dominic Christopher et AT BE CH CY DE DK ES FI FR GB GR IE IT LI LU al MC NL PT SE TR Harrison Goddard Foote LLP Designated Extension States: 4th Floor, Merchant Exchange RO SI 17-19 Whitworth Street West Manchester M1 5WG (GB) (30) Priority: 14.02.2001 GB 0103638 30.03.2001 US 280044 P (56) References cited: 05.04.2001 US 827158 WO-A-00/25127 WO-A-01/66089 11.05.2001 GB 0111597 WO-A-95/25504 WO-A1-02/32420 07.09.2001 GB 0121715 WO-A1-02/069993 US-A- 3 560 625 12.09.2001 US 951022 • EITAN ALHANATY; ET AL.: "Osmotic fragility of (43) Date of publication of application: liposomes as affected by antihemolytic 19.11.2003 Bulletin 2003/47 compounds" BIOCHIMICA ET BIOPHYSICA ACTA, vol. 339, no. 1, 1974, pages 146-155, (60) Divisional application: XP008008726 10180611.5 / 2 286 793 • PATRICIA S. -

Student Members of the American Chemical Society Poster Presentation Event November 6, 2020 5:30 - 7:00 Pm Stonecipher Lecture Hall
Student Members of the American Chemical Society Poster Presentation Event November 6, 2020 5:30 - 7:00 pm Stonecipher Lecture Hall Author: Brooke Underwood*, Courtney LaPointe, Dr. Jeffrey Boles Contact information: [email protected] Title: Liquid-liquid extraction and ultraviolet visible spectroscopy methods for distinguishing between hemp and marijuana Abstract: In December 2018, cannabis containing less than 0.3% tetrahydrocannabinol (THC), otherwise known as hemp, became legalized due to passage of the Farm Bill (1). This creates problems for law enforcement since current presumptive test kits either 1) don’t work at all or 2) work somewhat in differentiating between legal and illegal hemp crops. This problem exists because most hemp crops and hemp products contain low levels of THC and the carboxylated form, THCA. Our approach involves the advancement of an efficient, mobile, liquid-liquid extraction (LLE) that provides presumptive, qualitative forensic evidence of the chemical extract of a bud or other plant material. This research is focused on developing a kit that functions in a similar manner to NIK kits, commonly used by law enforcement, where all components of the kit are contained within a bag. The current NIK kit for Marijuana provides a false positive when Hemp is placed in the bag, thus creating the need for a more reliable test (2). The evidence would later be sent to a crime lab for definitive analysis and quantitation of THC by ultraviolet-visible spectroscopy (UV-vis). This research has focused on the utilization of liquid-liquid extraction techniques and commercially available stains. The methods presented are rapid (requiring no more than five to six minutes to complete). -

Vaginal Administration of Contraceptives
Scientia Pharmaceutica Review Vaginal Administration of Contraceptives Esmat Jalalvandi 1,*, Hafez Jafari 2 , Christiani A. Amorim 3 , Denise Freitas Siqueira Petri 4 , Lei Nie 5,* and Amin Shavandi 2,* 1 School of Engineering and Physical Sciences, Heriot-Watt University, Edinburgh EH14 4AS, UK 2 BioMatter Unit, École Polytechnique de Bruxelles, Université Libre de Bruxelles, Avenue F.D. Roosevelt, 50-CP 165/61, 1050 Brussels, Belgium; [email protected] 3 Pôle de Recherche en Gynécologie, Institut de Recherche Expérimentale et Clinique, Université Catholique de Louvain, 1200 Brussels, Belgium; [email protected] 4 Fundamental Chemistry Department, Institute of Chemistry, University of São Paulo, Av. Prof. Lineu Prestes 748, São Paulo 05508-000, Brazil; [email protected] 5 College of Life Sciences, Xinyang Normal University, Xinyang 464000, China * Correspondence: [email protected] (E.J.); [email protected] (L.N.); [email protected] (A.S.); Tel.: +32-2-650-3681 (A.S.) Abstract: While contraceptive drugs have enabled many people to decide when they want to have a baby, more than 100 million unintended pregnancies each year in the world may indicate the contraceptive requirement of many people has not been well addressed yet. The vagina is a well- established and practical route for the delivery of various pharmacological molecules, including contraceptives. This review aims to present an overview of different contraceptive methods focusing on the vaginal route of delivery for contraceptives, including current developments, discussing the potentials and limitations of the modern methods, designs, and how well each method performs for delivering the contraceptives and preventing pregnancy. -

Protocol for Look-Alike and Sound-Alike Drugs
Community Mental Health for Central Michigan PROTOCOL FOR LOOK-ALIKE AND SOUND-ALIKE DRUGS This protocol should be posted in all licensed residential group homes who contract with Community Mental Health for Central Michigan ADMINISTRATIVE GUIDELINE In an effort to improve medication safety and to meet The Joint Commission’s National Patient Safety Goal Number 3, Community Mental Health for Central Michigan (CMHCM) has identified a process to address sound-alike and look-alike drugs. The Performance Improvement Team that developed this guideline will continue to monitor new drugs on the market as they are released, review the list included with this guideline on an annual basis, and provide appropriate updates. Appropriate action to prevent errors involving the interchange of these drugs will be taken. Each CMHCM site (including direct and provider network sites) where medication is distributed or administered will post the attached lists and implement a plan for preventing drug mix-ups. This plan may consist of but not be limited to: Listing both the brand and generic names on medication records. Storing products with look-alike or sound-alike names in different locations. Employing double checks in the distribution process. Affixing “name alert” stickers to areas where look-alike or sound-alike products are stored. Changing the appearance of look-alike product names on pharmacy labels, computer screens, shelf labels and bins, and medication records by highlighting, through bold face, color, and/or tall man letters, the parts of the names that are different (e.g. hydrOXYzine, hydrALAzine). Having physicians write prescriptions using both the brand and generic names. -

Managing Opioid-Induced Constipation
Visual summary MODIFY PAIN RELIEF Managing opioid-induced constipation Is the current dose of opioid needed to control pain? Constipation affects many patients using opioids Could pain be relieved in other ways? to relieve pain associated with advanced cancer Non-opioid analgesics Interventional pain management or terminal disease. Constipation is often multifactorial in such patients, Could an opioid with less constipating effect be used? and opioids may be one of several causes. As well Buprenorphine Transdermal fentanyl as direct treatment of the constipation, adjusting medication regimens and treating exacerbating factors may help to provide relief from bowel transit Is a referral to other specialists needed? symptoms. Palliative care Neuro-gastroenterology Pain clinic ADDRESS EXACERBATING FACTORS DIRECTLY TREAT CONSTIPATION Drugs Non-pharmacological options 5HT3 antagonists Anticholinergics Opioids Increase fluid consumption Increase dietary fibres Antipsychotics Diuretics Iron Increase physical activity (within patients’ capabilities) Calcium supplements Calcium channel blockers Privacy and comfort during defecation Chemotherapies Complementary therapy Thalidomide Vinca alkaloids Positioning on toilet (to relax the puborectalis muscle) knees higher than hips, leaning forward Busulfan Carboplatin with elbows on knees, straightened spine Manual removal Nutritional and if the constipation is severe and refractory to other therapies metabolic issues Pharmacological management Dehydration Reduced food and fluids intake Oral laxatives Poor -

2021 Formulary List of Covered Prescription Drugs
2021 Formulary List of covered prescription drugs This drug list applies to all Individual HMO products and the following Small Group HMO products: Sharp Platinum 90 Performance HMO, Sharp Platinum 90 Performance HMO AI-AN, Sharp Platinum 90 Premier HMO, Sharp Platinum 90 Premier HMO AI-AN, Sharp Gold 80 Performance HMO, Sharp Gold 80 Performance HMO AI-AN, Sharp Gold 80 Premier HMO, Sharp Gold 80 Premier HMO AI-AN, Sharp Silver 70 Performance HMO, Sharp Silver 70 Performance HMO AI-AN, Sharp Silver 70 Premier HMO, Sharp Silver 70 Premier HMO AI-AN, Sharp Silver 73 Performance HMO, Sharp Silver 73 Premier HMO, Sharp Silver 87 Performance HMO, Sharp Silver 87 Premier HMO, Sharp Silver 94 Performance HMO, Sharp Silver 94 Premier HMO, Sharp Bronze 60 Performance HMO, Sharp Bronze 60 Performance HMO AI-AN, Sharp Bronze 60 Premier HDHP HMO, Sharp Bronze 60 Premier HDHP HMO AI-AN, Sharp Minimum Coverage Performance HMO, Sharp $0 Cost Share Performance HMO AI-AN, Sharp $0 Cost Share Premier HMO AI-AN, Sharp Silver 70 Off Exchange Performance HMO, Sharp Silver 70 Off Exchange Premier HMO, Sharp Performance Platinum 90 HMO 0/15 + Child Dental, Sharp Premier Platinum 90 HMO 0/20 + Child Dental, Sharp Performance Gold 80 HMO 350 /25 + Child Dental, Sharp Premier Gold 80 HMO 250/35 + Child Dental, Sharp Performance Silver 70 HMO 2250/50 + Child Dental, Sharp Premier Silver 70 HMO 2250/55 + Child Dental, Sharp Premier Silver 70 HDHP HMO 2500/20% + Child Dental, Sharp Performance Bronze 60 HMO 6300/65 + Child Dental, Sharp Premier Bronze 60 HDHP HMO -

Your 2017 Three-Tier
Your 2017 Three-Tier Prescription Drug List effective January 1, 2017 Connecticut Advantage Three-Tier Please read: This document contains information about commonly prescribed medications. This Prescription Drug List (PDL) is accurate as of January 1, 2017 and is subject to change after this date. The next anticipated update will be in July 2017. Your estimated coverage and copay/co-insurance may vary based on the benefit plan you choose and the effective date of the plan. For additional information: Call the toll-free member phone number on your health plan ID card. Visit myuhc.com® • Locate a participating retail pharmacy by ZIP code. • Look up possible lower-cost medication alternatives. • Compare medication pricing and options. 1 Your Prescription Drug List This Prescription Drug List (PDL) outlines covered medications for certain conditions and organizes them into cost levels, also known as tiers. An important part of the PDL is giving you choices so you and your doctor can choose the best course of treatment for you. Go to myuhc.com® for complete drug information Since the PDL may change, we encourage you to visit our website, myuhc.com. This website is the best source for up-to-date information about the medications your pharmacy benefit covers, possible lower-cost options, and cost comparisons. To view PDL information without logging on to the member portal, visit myuhc.com and select “Pharmacy Information” under “Links and Tools.” 2 At UnitedHealthcare, we want to help you better understand your medication options. Your pharmacy benefit offers flexibility and choice in determining the right medication for you. -

World Health Organization Model List of Essential Medicines, 21St List, 2019
World Health Organizatio n Model List of Essential Medicines 21st List 2019 World Health Organizatio n Model List of Essential Medicines 21st List 2019 WHO/MVP/EMP/IAU/2019.06 © World Health Organization 2019 Some rights reserved. This work is available under the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 IGO licence (CC BY-NC-SA 3.0 IGO; https://creativecommons.org/licenses/by-nc-sa/3.0/igo). Under the terms of this licence, you may copy, redistribute and adapt the work for non-commercial purposes, provided the work is appropriately cited, as indicated below. In any use of this work, there should be no suggestion that WHO endorses any specific organization, products or services. The use of the WHO logo is not permitted. If you adapt the work, then you must license your work under the same or equivalent Creative Commons licence. If you create a translation of this work, you should add the following disclaimer along with the suggested citation: “This translation was not created by the World Health Organization (WHO). WHO is not responsible for the content or accuracy of this translation. The original English edition shall be the binding and authentic edition”. Any mediation relating to disputes arising under the licence shall be conducted in accordance with the mediation rules of the World Intellectual Property Organization. Suggested citation. World Health Organization Model List of Essential Medicines, 21st List, 2019. Geneva: World Health Organization; 2019. Licence: CC BY-NC-SA 3.0 IGO. Cataloguing-in-Publication (CIP) data. CIP data are available at http://apps.who.int/iris. -

EUROPEAN PHARMACOPOEIA 10.0 Index 1. General Notices
EUROPEAN PHARMACOPOEIA 10.0 Index 1. General notices......................................................................... 3 2.2.66. Detection and measurement of radioactivity........... 119 2.1. Apparatus ............................................................................. 15 2.2.7. Optical rotation................................................................ 26 2.1.1. Droppers ........................................................................... 15 2.2.8. Viscosity ............................................................................ 27 2.1.2. Comparative table of porosity of sintered-glass filters.. 15 2.2.9. Capillary viscometer method ......................................... 27 2.1.3. Ultraviolet ray lamps for analytical purposes............... 15 2.3. Identification...................................................................... 129 2.1.4. Sieves ................................................................................. 16 2.3.1. Identification reactions of ions and functional 2.1.5. Tubes for comparative tests ............................................ 17 groups ...................................................................................... 129 2.1.6. Gas detector tubes............................................................ 17 2.3.2. Identification of fatty oils by thin-layer 2.2. Physical and physico-chemical methods.......................... 21 chromatography...................................................................... 132 2.2.1. Clarity and degree of opalescence of -

810672 Rev 03 22 15:Dept Phys Order Template.Qxd.Qxd
UMASS MEMORIAL MEDICAL CENTER NAME: BIRTHDATE/AGE: SEX: PHYSICIAN’S ORDERS ADULT INTRAVENOUS MEDICAL RECORD NUMBER: PATIENT-CONTROLLED ANALGESIA (PCA) Page 1 of 4 ECD / ACCOUNT NUMBER: Height Weight Inches ________ Cm. ________ Lbs. ________ Kg. ________ ALLERGIES: YES (LIST BELOW) OR LISTED PREVIOUSLY PRINT CLEARLY IN INK OR IMPRINT WITH PATIENT’S CARD 3 NONE KNOWN PROVIDER TO SIGN AND PLACE PAGER NUMBER LEGIBLY UNDER EACH ORDER SET INDICATE CHOICE OF ORDER OPTIONS BY USING X IN CHECK BOXES Attending/Change Attending To: __________________________________________________________________________ Pager: ________ (First) (Last) Resident: __________________________________ Pager: __________ Overnight coverage: ______________________ Pager: ________ Intern/NP/PA (First Call): ______________________ Pager: __________ House Staff Coverage: Yes No (uncovered) ALL OTHER ORDERS DATE TIME MEDICATION ORDERS ONLY 1. Assess pain and sedation level as per hospital policy, using Discontinue all previous opioids and benzodiazepines; additional appropriate tools (e.g. POSS, RASS). opioids and benzodiazepines must be re-ordered with initiation of PCA 2. Obtain vital signs, pain, and sedation levels prior to initiation or change in PCA. Then monitor vital signs, pain, and sedation 1. Choose drug, dosing category, PCA dose* levels every 15 min x 4, every hour x 4, then every 4 hours. (CHOOSE ONLY ONE DOSE CATEGORY): 3. Monitor continuous pulse oximetry for first 24 hrs. Page PCA Standard Dose : ordering team thereafter to continue monitoring as needed. Morphine 1 mg/mL 4. Call MD/LIP for: PCA dose: ___ mg (usual dose 1mg, range 0.5 - 5mg) Respiratory rate < 10 or SpO2 < 93% HYDROmorphone 0.2 mg/mL Unsatisfactory analgesia > 1 hour from previous adjustment PCA dose: ___ mg (usual dose 0.2mg, range 0.1 - 1.4mg) Increasing sedation (POSS score > 3 or RASS < 0) FentaNYL 20 mcg/mL Unsatisfactorily treated nausea/vomiting or pruritus PCA dose: ___ mcg (usual dose 10 mcg, range 10 - 50mcg) 5. -
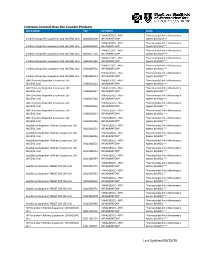
Common Covered Over-The-Counter Products Last Updated 08/25/20
Common Covered Over-the-Counter Products DRUG NAME NDC CATEGORY CLASS *ANALGESICS - ANTI- *Nonsteroidal Anti-inflammatory Childrens Ibuprofen Suspension 100 MG/5ML Oral 00904530909 INFLAMMATORY* Agents (NSAIDs)*** *ANALGESICS - ANTI- *Nonsteroidal Anti-inflammatory Childrens Ibuprofen Suspension 100 MG/5ML Oral 00904530920 INFLAMMATORY* Agents (NSAIDs)*** *ANALGESICS - ANTI- *Nonsteroidal Anti-inflammatory Childrens Ibuprofen Suspension 100 MG/5ML Oral 00904557720 INFLAMMATORY* Agents (NSAIDs)*** *ANALGESICS - ANTI- *Nonsteroidal Anti-inflammatory Childrens Ibuprofen Suspension 100 MG/5ML Oral 45802013326 INFLAMMATORY* Agents (NSAIDs)*** *ANALGESICS - ANTI- *Nonsteroidal Anti-inflammatory Childrens Ibuprofen Suspension 100 MG/5ML Oral 45802089726 INFLAMMATORY* Agents (NSAIDs)*** *ANALGESICS - ANTI- *Nonsteroidal Anti-inflammatory Childrens Ibuprofen Suspension 100 MG/5ML Oral 45802089734 INFLAMMATORY* Agents (NSAIDs)*** GNP Childrens Ibuprofen Suspension 100 *ANALGESICS - ANTI- *Nonsteroidal Anti-inflammatory MG/5ML Oral 24385036126 INFLAMMATORY* Agents (NSAIDs)*** GNP Childrens Ibuprofen Suspension 100 *ANALGESICS - ANTI- *Nonsteroidal Anti-inflammatory MG/5ML Oral 24385036134 INFLAMMATORY* Agents (NSAIDs)*** GNP Childrens Ibuprofen Suspension 100 *ANALGESICS - ANTI- *Nonsteroidal Anti-inflammatory MG/5ML Oral 24385037226 INFLAMMATORY* Agents (NSAIDs)*** GNP Childrens Ibuprofen Suspension 100 *ANALGESICS - ANTI- *Nonsteroidal Anti-inflammatory MG/5ML Oral 24385090526 INFLAMMATORY* Agents (NSAIDs)*** GNP Childrens Ibuprofen Suspension