Flotation Separation of Scheelite from Fluorite Using Sodium Polyacrylate As Inhibitor
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Review of Flotation Separation of Mg Carbonates (Dolomite and Magnesite)
minerals Review A Review of Flotation Separation of Mg Carbonates (Dolomite and Magnesite) Darius G. Wonyen 1,†, Varney Kromah 1,†, Borbor Gibson 1,† ID , Solomon Nah 1,† and Saeed Chehreh Chelgani 1,2,* ID 1 Department of Geology and Mining Engineering, Faculty of Engineering, University of Liberia, P.O. Box 9020 Monrovia, Liberia; [email protected] (D.G.W.); [email protected] (Y.K.); [email protected] (B.G.); [email protected] (S.N.) 2 Department of Electrical Engineering and Computer Science, University of Michigan, Ann Arbor, MI 48109, USA * Correspondence: [email protected]; Tel.: +1-41-6830-9356 † These authors contributed equally to the study. Received: 24 July 2018; Accepted: 13 August 2018; Published: 15 August 2018 Abstract: It is well documented that flotation has high economic viability for the beneficiation of valuable minerals when their main ore bodies contain magnesium (Mg) carbonates such as dolomite and magnesite. Flotation separation of Mg carbonates from their associated valuable minerals (AVMs) presents several challenges, and Mg carbonates have high levels of adverse effects on separation efficiency. These complexities can be attributed to various reasons: Mg carbonates are naturally hydrophilic, soluble, and exhibit similar surface characteristics as their AVMs. This study presents a compilation of various parameters, including zeta potential, pH, particle size, reagents (collectors, depressant, and modifiers), and bio-flotation, which were examined in several investigations into separating Mg carbonates from their AVMs by froth flotation. Keywords: dolomite; magnesite; flotation; bio-flotation 1. Introduction Magnesium (Mg) carbonates (salt-type minerals) are typical gangue phases associated with several valuable minerals, and have complicated processing [1,2]. -

Overview of Tungsten Indicator Minerals Scheelite and Wolframite with Examples from the Sisson W-Mo Deposit, Canada
Overview of tungsten indicator minerals scheelite and wolframite with examples from the Sisson W-Mo deposit, Canada M. Beth McClenaghan1, M.A. Parkhill2, A.A. Seaman3, A.G. Pronk3, M. McCurdy1 & D.J. Kontak4 1Geological Survey of Canada, 601 Booth Street, Ottawa, Ontario, Canada K1A 0E8 (e-mail: [email protected]) 2New Brunswick Department of Energy and Mines, Geological Surveys Branch, P.O. Box 50, Bathurst, New Brunswick, Canada E2A 3Z1 3New Brunswick Department of Energy and Mines, Geological Surveys Branch, P.O. Box 6000, Fredericton, New Brunswick, Canada E3B 5H1 4Department of Earth Sciences, Laurentian University, Sudbury, Ontario, Canada P3E 2C6 These short course notes provide an overview of published lit- Table 1. List of regional surveys and case studies conducted around erature on the use of scheelite and wolframite as indicator min- the world in which scheelite and/or wolframite in surficial sediments erals for W, Mo, and Au exploration. The use of scheelite and have been used as indicator minerals. wolframite in stream sediments is well documented for mineral Mineral Media Location Source of Information exploration but less so for using glacial sediments (Table 1). scheelite stream sediments Pakistan Asrarullah (1982) wolframite stream sediments Burma ESCAP Scretariat (1982) The Geological Survey of Canada has recently conducted a scheelite, wolframite stream sediments USA Theobald & Thompson (1960) glacial till and stream sediment indicator mineral case study scheelite stream sediments, soil Thailand Silakul (1986) around the Sisson W-Mo deposit in eastern Canada. scheelite stream sediments Greenland Hallenstein et al. (1981) Preliminary indicator mineral results from this ongoing study scheelite stream sediments Spain Fernández-Turiel et al. -

Geology of Tungsten Deposits in North-Central Chile
UNITED STATES DEPARTMENT OF THE INTERIOR J. A. Krug, Secretary GEOLOGICAL SURVEY W. E. Wrather, Director Bulletin 960-C GEOLOGY OF TUNGSTEN DEPOSITS IN NORTH-CENTRAL CHILE BY JAMES F. McALLISTER AND CARLOS RUIZ F. Prepared in cooperation with the DEPARTAMENTO DE MINAS Y PETROLED DE CHILE under the auspices of INTERDEPARTMENTAL COMMITTEE ON SCIENTIFIC AND CULTURAL COOPERATION, DEPARTMENT OF STATE Geologic Investigations in the American Republics, 1947 (Pages 89-108) UNITED STATES GOVERNMENT PRINTING OFFICE WASHINGTON : 1948 For sale by the Superintendent of Documents, U. S. Government Printing Office, Washington 25, D. C. Price $1.75 CONTENTS Page Abstract____..______________________________._ 89 Introduction___________________________-__________ __________-___ 89 Geology_________________________________..._ 92 Rocks.____________________________________ 92 Structure____________________________________ 92 Minerals. _.---_.----_..---_.------_- _- -- 92 Type, size, and grade of ore deposits.------------._-----_-_-_-_-_ 93 Descriptions of mining properties__________-___-_-_-_---__--_---____- 94 Salamanca region____________________________________________ 94 Castaneda de Llamuco mine--___----------_-------_------__ 94 Picbe prospect... ________.- _ . 97 Domeyko region_______________________________________________ 97 El Durazno district.-..__-___.___--_____-__________...___ 97 Boliviana mine-..._________-____-._--._._-__-_--__-__. 99 Minillas property......____________________________________ 99 Vallenar region______________________________________________ 101 San Antonio mining property.______________________________ 102 Zapallo de Chehuque mining property_________*____-______- 104 Metric equivalents____-____-_____-_______-----_-__--_-_-_-__--_-- 106 Index.--.___-____-_._______________._______________ 107 Page PLATE 21. Geologic map of the Castaneda de Llamuco tungsten deposit. In pocket 22. Plan of workings, Castafieda de Llamuco mine. _-____-___ In pocket 23. -

Implications for Environmental and Economic Geology
UNIVERSITY OF CALIFORNIA RIVERSIDE Kinetics of the Dissolution of Scheelite in Groundwater: Implications for Environmental and Economic Geology A Thesis submitted in partial satisfaction of the requirements for the degree of Master of Science in Geological Sciences by Stephanie Danielle Montgomery March 2013 Thesis Committee: Dr. Michael A. McKibben, Chairperson Dr. Christopher Amrhein Dr. Timothy Lyons Copyright by Stephanie Danielle Montgomery 2013 The Thesis of Stephanie Danielle Montgomery is approved: _________________________________________ _________________________________________ _________________________________________ Committee Chairperson University of California, Riverside ABSTRACT OF THE THESIS Kinetics of the Dissolution of Scheelite in Groundwater: Implications for Environmental and Economic geology by Stephanie Danielle Montgomery Masters of Science, Graduate Program in Geological Sciences University of California, Riverside, March 2013 Dr. Michael McKibben, Chairperson Tungsten, an emerging contaminant, has no EPA standard for its permissible levels in drinking water. At sites in California, Nevada, and Arizona there may be a correlation between elevated levels of tungsten in drinking water and clusters of childhood acute lymphocytic leukemia (ALL). Developing a better understanding of how tungsten is released from rocks into surface and groundwater is therefore of growing environmental interest. Knowledge of tungstate ore mineral weathering processes, particularly the rates of dissolution of scheelite (CaWO4) in groundwater, could improve models of how tungsten is released and transported in natural waters. Our research focused on the experimental determination of the rates and products of scheelite dissolution in 0.01 M NaCl (a proxy for groundwater), as a function of temperature, pH, and mineral surface area. Batch reactor experiments were conducted within constant temperature circulation baths over a pH range of 3-10.5. -

Abstract Spectroscopic Characterization of Fluorite
ABSTRACT SPECTROSCOPIC CHARACTERIZATION OF FLUORITE: RELATIONSHIPS BETWEEN TRACE ELEMENT ZONING, DEFECTS AND COLOR By Carrie Wright This thesis consists of two separate papers on color in fluorite. In the first paper, synthetic fluorites doped with various REEs (10-300 ppm) were analyzed using direct current plasma spectrometry, optical absorption spectroscopy, fluorescence spectrophotometry, and electron paramagnetic resonance spectroscopy before and after receiving 10-25 Mrad of 60Co gamma irradiation. The combined results of these techniques indicate that the irradiation-induced color of the Y-, Gd-, La- and Ce-doped samples are the result of a REE-associated fluorine vacancy that traps two electrons. Divalent samarium may be the cause of the irradiation-induced green color of the Sm- doped sample. In the second paper, fluorite crystals from Bingham, NM, Long Lake, NY, and Westmoreland, NH were similarly investigated to determine the relationship between sectorally zoned trace elements, defects, and color. The results indicate causes of color similar to those in the synthetic samples with the addition of simple F-centers. SPECTROSCOPIC CHARACTERIZATION OF FLUORITE: RELATIONSHIPS BETWEEN TRACE ELEMENT ZONING, DEFECTS AND COLOR A Thesis Submitted to the Faculty of Miami University In partial fulfillment of The requirements for the degree of Master of Science Department of Geology By Carrie Wright Miami University Oxford, OH 2002 Advisor_____________________ Dr. John Rakovan Reader______________________ Dr. Hailiang Dong TABLE OF CONTENTS Chapter 1: Introduction to the cause of color in fluorite 1 Manuscript 1-Chapter 2 29 “Spectroscopic investigation of lanthanide doped CaF2 crystals: implications for the cause of color” Manuscript 2-Chapter 3 95 “Spectroscopic characterization of fluorite from Bingham, NM, Long Lake, NY and Westmoreland, NH: relationships between trace element zoning, defects and color ii TABLE OF FIGURES Chapter 1 Figures 21 Figure 1a. -
Mineral Identification Chart – LECTURE
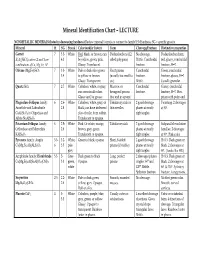
Mineral Identification Chart – LECTURE NONMETALLIC MINERALS (listed in decreasing hardness) Review mineral formula to connect to family! H=Hardness; SG = specific gravity Mineral H SG Streak Color (and/or luster) Form Cleavage/Fracture Distinctive properties Garnet 7 3.5- White Red, black, or brown; can Dodecahedrons (12- No cleavage. Dodecahedron form, X3Y2(SiO4)3 where X and Y are 4.3 be yellow, green, pink. sided polygons) Brittle. Conchoidal red, glassy, conchoidal combinations of Ca, Mg, Fe, Al Glassy. Translucent. fracture. fracture, H=7. Olivine (Mg,Fe)2SiO4 7 3.3- White Pale or dark olive green Short prisms Conchoidal Green, conchoidal 3.4 to yellow or brown. (usually too small to fracture. fracture, glassy, H=7. Glassy. Transparent. see). Brittle. Usually granular. Quartz SiO2 7 2.7 White Colorless, white, or gray; Massive; or Conchoidal Glassy, conchoidal can occur in all colors. hexagonal prisms fracture. fracture, H=7. Hex. Glassy and/or greasy. that end in a point. prism with point end. Plagioclase Feldspar family: 6 2.6- White Colorless, white, gray, or Tabular crystals or 2 good cleavage Twinning. 2 cleavages Anorthite and Labradorite 2.8 black; can have iridescent thin needles planes at nearly at 90°. CaAl2Si2O8 to Oligoclase and play of color from within. right angles. Albite NaAlSi3O8 Translucent to opaque. Potassium Feldspar family: 6 2.5- White Pink. Or white, orange, Tabular crystals 2 good cleavage Subparallel exsolution Orthoclase and Microcline 2.6 brown, gray, green. planes at nearly lamellae. 2 cleavages KAlSi3O8 Translucent to opaque. right angles. at 90°. Pink color. Pyroxene family: Augite 5.5- 3.2- White, Green to black; opaque. -

Role of Sodium Carbonate in Scheelite Flotation – a Multi-Faceted Reagent
Helmholtz-Zentrum Dresden-Rossendorf (HZDR) Role of sodium carbonate in scheelite flotation – a multi-faceted reagent Kupka, N.; Rudolph, M.; Originally published: October 2018 Minerals Engineering 129(2018), 120-128 DOI: https://doi.org/10.1016/j.mineng.2018.09.005 Perma-Link to Publication Repository of HZDR: https://www.hzdr.de/publications/Publ-27114 Release of the secondary publication on the basis of the German Copyright Law § 38 Section 4. CC-BY-NC-ND 4.0 *Manuscript Click here to view linked References Role of sodium carbonate in scheelite flotation – a multi-faceted reagent 1 1,a 1 2 Nathalie Kupka ; Martin Rudolph 3 1 Helmholtz Institute Freiberg for Resource Technology, Helmholtz Zentrum Dresden-Rossendorf, Chemnitzer 4 Straße 40, 09599 Freiberg, Germany 5 a 6 [email protected] 7 8 Abstract 9 10 Even though sodium carbonate is a reagent frequently used in flotation, its role is mostly described as 11 a buffering pH modifier and a pulp dispersant. In the case of scheelite flotation, it has been hinted that 12 13 sodium carbonate improves both grade and/or recovery but the mechanism itself is ambiguous at best 14 or at least has not been distinctly reported in the literature. Furthermore, the addition of depressants 15 such as sodium silicate or quebracho could be triggering additional mechanisms. Through batch 16 flotation testwork on a skarn scheelite ore with high calcite content, single mineral flotation and 17 contact angle measurements, this article aims at demonstrating that sodium carbonate is a multi- 18 faceted reagent, which serves as a buffering pH modifier, a pulp dispersant precipitating calcium and 19 20 magnesium ions in suspension, a depressant for calcite and calcium silicates and also a promoter for 21 scheelite. -

Winter 1998 Gems & Gemology
WINTER 1998 VOLUME 34 NO. 4 TABLE OF CONTENTS 243 LETTERS FEATURE ARTICLES 246 Characterizing Natural-Color Type IIb Blue Diamonds John M. King, Thomas M. Moses, James E. Shigley, Christopher M. Welbourn, Simon C. Lawson, and Martin Cooper pg. 247 270 Fingerprinting of Two Diamonds Cut from the Same Rough Ichiro Sunagawa, Toshikazu Yasuda, and Hideaki Fukushima NOTES AND NEW TECHNIQUES 281 Barite Inclusions in Fluorite John I. Koivula and Shane Elen pg. 271 REGULAR FEATURES 284 Gem Trade Lab Notes 290 Gem News 303 Book Reviews 306 Gemological Abstracts 314 1998 Index pg. 281 pg. 298 ABOUT THE COVER: Blue diamonds are among the rarest and most highly valued of gemstones. The lead article in this issue examines the history, sources, and gemological characteristics of these diamonds, as well as their distinctive color appearance. Rela- tionships between their color, clarity, and other properties were derived from hundreds of samples—including such famous blue diamonds as the Hope and the Blue Heart (or Unzue Blue)—that were studied at the GIA Gem Trade Laboratory over the past several years. The diamonds shown here range from 0.69 to 2.03 ct. Photo © Harold & Erica Van Pelt––Photographers, Los Angeles, California. Color separations for Gems & Gemology are by Pacific Color, Carlsbad, California. Printing is by Fry Communications, Inc., Mechanicsburg, Pennsylvania. © 1998 Gemological Institute of America All rights reserved. ISSN 0016-626X GIA “Cut” Report Flawed? The long-awaited GIA report on the ray-tracing analysis of round brilliant diamonds appeared in the Fall 1998 Gems & Gemology (“Modeling the Appearance of the Round Brilliant Cut Diamond: An Analysis of Brilliance,” by T. -

Porphyry Deposits
PORPHYRY DEPOSITS W.D. SINCLAIR Geological Survey of Canada, 601 Booth St., Ottawa, Ontario, K1A 0E8 E-mail: [email protected] Definition Au (±Ag, Cu, Mo) Mo (±W, Sn) Porphyry deposits are large, low- to medium-grade W-Mo (±Bi, Sn) deposits in which primary (hypogene) ore minerals are dom- Sn (±W, Mo, Ag, Bi, Cu, Zn, In) inantly structurally controlled and which are spatially and Sn-Ag (±W, Cu, Zn, Mo, Bi) genetically related to felsic to intermediate porphyritic intru- Ag (±Au, Zn, Pb) sions (Kirkham, 1972). The large size and structural control (e.g., veins, vein sets, stockworks, fractures, 'crackled zones' For deposits with currently subeconomic grades and and breccia pipes) serve to distinguish porphyry deposits tonnages, subtypes are based on probable coproduct and from a variety of deposits that may be peripherally associat- byproduct metals, assuming that the deposits were econom- ed, including skarns, high-temperature mantos, breccia ic. pipes, peripheral mesothermal veins, and epithermal pre- Geographical Distribution cious-metal deposits. Secondary minerals may be developed in supergene-enriched zones in porphyry Cu deposits by weathering of primary sulphides. Such zones typically have Porphyry deposits occur throughout the world in a series significantly higher Cu grades, thereby enhancing the poten- of extensive, relatively narrow, linear metallogenic tial for economic exploitation. provinces (Fig. 1). They are predominantly associated with The following subtypes of porphyry deposits are Mesozoic to Cenozoic orogenic belts in western North and defined according to the metals that are essential to the eco- South America and around the western margin of the Pacific nomics of the deposit (metals that are byproducts or poten- Basin, particularly within the South East Asian Archipelago. -

Stable Isotope Studies of Quartz-Vein Type Tungsten Deposits in Dajishan Mine, Jiangxi Province, Southeast China
Stable Isotope Geochemistry: A Tribute to Samuel Epstein © The Geochemical Society, Special Publication No.3, 1991 Editors: H. P. Taylor, Jr., J. R. O'Neil and I. R. Kaplan Stable isotope studies of quartz-vein type tungsten deposits in Dajishan Mine, Jiangxi Province, Southeast China YUCH-NING SHIEH Department of Earth and Atmospheric Sciences, Purdue University, West Lafayette, IN 47907, U.S.A. and GUO-XIN ZHANG Institute of Geochemistry (Guangzhou Branch), Academia Sinica, Guangzhou, Guangdong 510640, People's Republic of China Abstract-The Dajishan tungsten deposits belong to the wolframite-quartz vein type. These occur as fissure-fillings in the contact zone between the Jurassic Yenshanian granites and Cambrian meta- sandstones and slates. Quartz, beryl, muscovite, wolframite, scheelite, and sulfides are the major minerals formed in the main stage of mineralization. Late-stage minerals include calcite, dolomite, quartz, fluorite, and scheelite (replacing wolframite). No granitic rocks are exposed at the surface, but drilling has revealed hidden granitic bodies ranging in composition from biotite granite and two- mica granite to muscovite granite and pegmatite. These may represent a differentiation series from a common magma. Exceedingly uniform 0180 values are found in the minerals from the main-stage veins: quartz = 11.1-12.7 (n = 27), muscovite = 8.4-10.0 (n = 22), wolframite = 4.1-5.3 (n = 15), scheelite = 4.2-5.5 (n = 4), suggesting that isotopic equilibrium apparently has attained and that relatively constant physico-chemical conditions prevailed throughout the main-stage of mineralization. Oxygen isotope fractionations for .quartz-wolframite, quartz-scheelite, and quartz-muscovite pairs give concordant isotopic temperatures of 320-390°C, consistent with results from fluid inclusion 018 oD studies. -

Mineralogy and Chemistry of Rare Earth Elements in Alkaline Ultramafic Rocks and Fluorite in the Western Kentucky Fluorspar District Warren H
Mineralogy and Chemistry of Rare Earth Elements in Alkaline Ultramafic Rocks and Fluorite in the Western Kentucky Fluorspar District Warren H. Anderson Report of Investigations 8 doi.org/10.13023/kgs.ri08.13 Series XIII, 2019 Cover Photo: Various alkaline ultramafic rocks showing porphyritic, brecciated, and aphanitic textures, in contact with host limestone and altered dike texture. From left to right: • Davidson North dike, Davidson core, YH-04, 800 ft depth. Lamprophyre with calcite veins, containing abundant rutile. • Coefield area, Billiton Minner core BMN 3. Intrusive breccia with lamprophyric (al- nöite) matrix. • Maple Lake area, core ML-1, 416 ft depth. Lamprophyre intrusive. • Maple Lake area, core ML-2, 513 ft depth. Lamprophyre (bottom) in contact with host limestone (top). Kentucky Geological Survey University of Kentucky, Lexington Mineralogy and Chemistry of Rare Earth Elements in Alkaline Ultramafic Rocks and Fluorite in the Western Kentucky Fluorspar District Warren H. Anderson Report of Investigations 8 doi.org/10.13023/kgs.ri08.13 Series XIII, 2019 Our Mission The Kentucky Geological Survey is a state-supported research center and public resource within the University of Kentucky. Our mission is to sup- port sustainable prosperity of the commonwealth, the vitality of its flagship university, and the welfare of its people. We do this by conducting research and providing unbiased information about geologic resources, environmen- tal issues, and natural hazards affecting Kentucky. Earth Resources—Our Common Wealth www.uky.edu/kgs © 2019 University of Kentucky For further information contact: Technology Transfer Officer Kentucky Geological Survey 228 Mining and Mineral Resources Building University of Kentucky Lexington, KY 40506-0107 Technical Level General Intermediate Technical Statement of Benefit to Kentucky Rare earth elements are used in many applications in modern society, from cellphones to smart weapons systems. -

Tungsten Minerals and Deposits
DEPARTMENT OF THE INTERIOR FRANKLIN K. LANE, Secretary UNITED STATES GEOLOGICAL SURVEY GEORGE OTIS SMITH, Director Bulletin 652 4"^ TUNGSTEN MINERALS AND DEPOSITS BY FRANK L. HESS WASHINGTON GOVERNMENT PRINTING OFFICE 1917 ADDITIONAL COPIES OF THIS PUBLICATION MAY BE PROCURED FROM THE SUPERINTENDENT OF DOCUMENTS GOVERNMENT PRINTING OFFICE WASHINGTON, D. C. AT 25 CENTS PER COPY CONTENTS. Page. Introduction.............................................................. , 7 Inquiries concerning tungsten......................................... 7 Survey publications on tungsten........................................ 7 Scope of this report.................................................... 9 Technical terms...................................................... 9 Tungsten................................................................. H Characteristics and properties........................................... n Uses................................................................. 15 Forms in which tungsten is found...................................... 18 Tungsten minerals........................................................ 19 Chemical and physical features......................................... 19 The wolframites...................................................... 21 Composition...................................................... 21 Ferberite......................................................... 22 Physical features.............................................. 22 Minerals of similar appearance.................................