Chemical and Bacteriological Status of Yeoti Lake of Mohol (Maharashtra)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

AQAR Report 2017-18
NATIONAL ASSESSMENT AND ACCREDITATION COUNCIL An Autonomous Institution of the University Grants Commission P. O. Box. No. 1075, Opp: NLSIU, Nagarbhavi, Bangalore - 560 072 India ANNUAL QUALITY ASSURANCE REPORT (AQAR) FOR THE YEAR 2017-18 Of WALCHAND COLLEGE OF ARTS & SCIENCE, SOLAPUR NAAC Track ID MHCOGN11529 1 Contents Page Nos. 1. Introduction ..... 3 2. Objective ...... 3 3. Strategies ...... 3 4. Functions ...... 4 5. Benefits ...... 4 6. Composition of the IQAC ...... 5 7. The role of coordinator ...... 6 8. Operational Features of the IQAC ...... 6 9. Monitoring Mechanism ...... 7 10. The Annual Quality Assurance Report (AQAR) of the IQAC ...... 8 Part – A 11. Details of the Institution ...... 8 12. IQAC Composition and Activities ...... 11 Part – B 13. Criterion – I: Curricular Aspects ...... 17 14. Criterion – II: Teaching, Learning and Evaluation ...... 19 15. Criterion – III: Research, Consultancy and Extension ...... 22 16. Criterion – IV: Infrastructure and Learning Resources ...... 33 17. Criterion – V: Student Support and Progression ...... 36 18. Criterion – VI: Governance, Leadership and Management ...... 44 19. Criterion – VII: Innovations and Best Practices ...... 51 20. Abbreviations ...... 57 21. Annexure i …… 58 22. Annexure ii …… 65 23. Annexure iii …… 66 ___________________________ Document revised by: Dr. Ganesh Hegde, Assistant Adviser and B. S. Ponmudiraj, Assistant Adviser, NAAC 2 Guidelines for the Creation of the Internal Quality Assurance Cell (IQAC) and Submission of Annual Quality Assurance Report (AQAR) in Accredited Institutions Introduction In pursuance of its Action Plan for performance evaluation, assessment and accreditation and quality up-gradation of institutions of higher education, the National Assessment and Accreditation Council (NAAC), Bangalore proposes that every accredited institution should establish an Internal Quality Assurance Cell (IQAC) as a post-accreditation quality sustenance measure. -

Ecosystem : an Ecosystem Is a Complete Community of Living Organisms and the Nonliving Materials of Their Surroundings
Solapur: Introduction: Solapur District is a district in Maharashtra state of India. The city of Solapur is the district headquarters. It is located on the south east edge of the state and lies entirely in the Bhima and Seena basins. Facts District - Solapur Area - 14886 km² Sub-divisions - Solapur, Madha (Kurduwadi), Pandharpur Talukas - North Solapur, Barshi, Akkalkot, South Solapur, Mohol,Mangalvedha, Pandharpur, Sangola, Malshiras, Karmala, Madha. Proposal for a separate Phandarpur District The Solapur district is under proposal to be bifurcated and a separate Phandarpur district be carved out of existing Solapur district. Distance from Mumbai - 450 km Means of transport - Railway stations -Solapur, Mohol, Kurduwadi, Madha, Akkalkot Road ST Buses, SMT (Solapur Municipal Transportation, Auto- Rikshaws. Solapur station has daily train service to Mumbai via Pune known as Siddheshwar Express Also, daily shuttle from Solapur to Pune known as Hutatma Express Population Total - 3,849,543(District) The district is 31.83% urban as of 2001. Area under irrigation - 4,839.15 km² Irrigation projects Major-1 Medium-2 Minor-69 Imp. Projs.- Bhima Ujjani Industries Big-98 Small-8986 Languages/dialects - Marathi, Kannada, Telagu Folk-Arts - Lavani, Gondhal, Dhangari,Aradhi and Bhalari songs Weather Temperature Max: 44.10 °C Min: 10.7 °C Rainfall-759.80 mm (Average) Main crops - Jowar, wheat, sugarcane Solapur district especially Mangalwedha taluka is known for Jowar. Maldandi Jowar is famous in all over Maharashtra. In December - January agriculturists celebrates Hurda Party. This is also famous event in Solapur. Hurda means pre-stage of Jowar. Agriculturists sow special breed of Hurda, named as Dudhmogra, Gulbhendi etc. -

Survey of Weeds As a Source of Pharmaceuticals from Mohol Tahasil
Global Journal of Science Frontier Research Agriculture & Biology Volume 12 Issue 3 Version 1.0 March 2012 Type : Double Blind Peer Reviewed International Research Journal Publisher: Global Journals Inc. (USA) Online ISSN: 2249-4626 & Print ISSN: 0975-5896 Survey of Weeds as a Source of Pharmaceuticals from Mohol Tahasil By Dalave S.C , Auti S.G & B.J. Apparao Padmashree Vikhe Patil College of Arts, Science and Commerce Pravaranagar Abstract – Weeds are considered as unwanted plants. However some weeds posses valuable pharmaceutical importance. Number of researchers combined this fact with an ethnobotanical approach. The present work reports, pharmaceutically important weeds from the common fields (Sorghum, Sugarcane, Maize, Wheat, Pulse Crop and waste land) of Mohol Tahasil of Solapur district, Maharashtra state. 21 valuable weed species were collected from different fields of Mohol Tahasil and identified for their pharmaceutical source using standard literature and herbal pharmacopoeias. Ethnomedicinal and weed survey in this area was not much explored and hence the present investigation was taken up. Keywords : Weeds, Pharmaceutical aspects. GJSFR -D Classification : FOR Code: 070308 Survey of Weeds as a Source of Pharmaceuticals from Mohol Tahasil Strictly as per the compliance and regulations of : © 2012 . Dalave S.C , Auti S.G & B.J. Apparao.This is a research/review paper, distributed under the terms of the Creative Commons Attribution-Noncommercial 3.0 Unported License http://creativecommons.org/licenses/by-nc/3.0/), permitting all non commercial use, distribution, and reproduction in any medium, provided the original work is properly cited. Survey of Weeds as a Source of Pharmaceuticals from Mohol Tahasil Dalave S.C Į, Auti S.Gı, & B.J. -

Review of Research Journal:International Monthly Scholarly
ISSN 2249-894X Impact Factor : 3.1402 (UIF) Volume - 5 | Issue - 3 | Dec - 2015 Review Of Research _________________________________________________________________________________ SOCIAL AUDIT OF NATIONAL RURAL EMPLOYMENT GUARANTEE SCHEME IN SOLAPUR DISTRICT Dr. S. V. Shinde Associate Professor , D. A. V. Velankar College of Commerce, Solapur. ABSTRACT : INTRODUCTION : National Rural Employment Guarantee Act 2005 (or, NREGA The act was first No 42, later renamed as the "Mahatma Gandhi National Rural proposed in 1991 by Narasimha Employment Guarantee Act", MGNREGA), is an Indian labour law Rao. In 2006, it was finally and social security measure that aims to guarantee the 'right to work'. accepted in the parliament and It aims to enhance livelihood security in rural areas by commenced implementation in providing at least 100 days of wage employment in a financial year to 200 districts of India. Based on every household whose adult members volunteer to do unskilled this pilot experience, NREGA manual work. was scoped up to covered all the districts of India from 1 April 2008. The MGNREGA was initiated with the objective of "enhancing livelihood security inrural areas by providing at least 100 days of guaranteed wage employment in a financial year, to every household whose adult members volunteer to do unskilled manual work". Another aim of MGNREGA is to create durable assets (such as roads, canals, ponds, wells). Employment is to be provided within 5 km of an applicant's residence, and minimum wages are to be paid. If work is not provided within 15 days of applying, applicants are entitled to an unemployment allowance. Thus, employment under MGNREGA is a legal entitlement. -
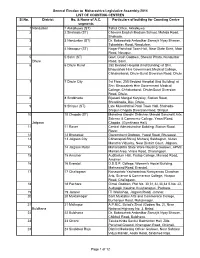
V E 2014 Counting Centres
General Election to Maharashtra Legislative Assembly-2014 LIST OF COUNTING CENTRES Sl.No. District No. & Name of A.C. Particulars of building for Counting Centre segments 1 Nandurbar 1 Akkalkuwa (ST) Tahsil Office, Akkalkuwa 2 2 Shahada (ST) Chavara English Medium School, Mohida Road, Shahada. 3 3 Nandurbar (ST) Dr. Babasaheb Ambedkar Samajik Nyay Bhavan, Tokartalav Road, Nandurbar. 4 4 Nawapur (ST) Nagar Parishad Town Hall, Near State Bank, Main Road, Navapur. 5 5 Sakri (ST) Govt. Grain Godown, Shewali Phata, Nandurbar Dhule Road, Sakri. 6 6 Dhule Rural 250 Bedded Hospital (Iind Building) at Shri. Bhausaheb Hire Government Medical College, Chhakarbardi, Dhule-Surat Diversion Road, Dhule. 7 7 Dhule City 1st Floor, 250 Bedded Hospital (Iind Building) at Shri. Bhausaheb Hire Government Medical College, Chhakarbardi, Dhule-Surat Diversion Road, Dhule. 8 8 Sindkheda Bijasani Mangal Karyalay, Station Road, Shindkheda, Dist. Dhule. 9 9 Shirpur (ST) Late Mukeshbhai Patel Town Hall, Shahada- Shirpur-Chopda Diversion Road, Shirpur. 10 10 Chopda (ST) Mahatma Gandhi Shikshan Mandal Sanchalit Arts, Science & Commerce College, Yawal Road, Jalgaon Chopda. (Gymkhana Hall) 11 11 Raver Central Administrative Building, Station Road, Raver. 12 12 Bhusawal Government Godown, Yawal Road, Bhusawal. 13 13 Jalgaon City Chhatrapati Shivaji Maharaj Sabhagruh, Nutan Maratha Vidyalay, Near District Court, Jalgaon. 14 14 Jalgaon Rural Maharashtra State Ware Housing Godown, APMC Market Area, Virare Road, Dharangaon. 15 15 Amalner Auditorium Hall, Pratap College, Marwad Road, Amalner. 16 16 Erandol D.D.S.P. College, Women's Hostel Building, Mahsawad Road, Erandol. 17 17 Chalisgaon Nanasaheb Yashwantrao Narayanrao Chawhan Arts, Science & Commerece College, Hirapur Road, Chalisgaon. -

Sr No Pc No Pc Name Ac No Ac Name Zonal Officer Name
DISTRICT COMMUNICATION PLAN SECTOR OFFICER DETAILS SR NO PC NO PC NAME AC NO AC NAME ZONAL OFFICER DESIGNATION AREA MOBILE NO LANDLINE EMAIL ID NAME COVERED 1 2 3 4 5 6 7 8 9 10 11 244 KARMALA 1 43 Madha 244 Karmala Kaustubh Dy.Eng. Bhima 1 To 9 9011032477 02182- Narendr Upasa Sinchan 222033 Saundane Sub.Div. No.1 245 KARMALA 2 43 Madha 244 Karmala S.G.Polake AgriKarmala Supervisor 10 To 14 , 9421031474 02182- [email protected] Taluka Agriculuter 36,77 220516 m Office Karmala 246 KARMALA 3 43 Madha 244 Karmala M.B.Nale Block Education 15 To 20, 34, 9975780099 02182- [email protected] Officer Panchayat 35, 78 221658 n Samiti Karmala 247 KARMALA 4 43 Madha 244 Karmala B P Patil I T I Karmala 21 To 26 , 33 9021182996 248 KARMALA 5 43 Madha 244 Karmala R B Darade Sectengr gramin Pani 27 To 32 95 9423202042 debn.karmala@rediffm Purwatha Karmala To 98 ail.com 249 KARMALA 6 43 Madha 244 Karmala J S Patil Z P Construction Sub 37 To 44 7083631579 02182- Division Karmala 220358 250 KARMALA 7 43 Madha 244 Karmala R C Bansode T Agri office,Karmala 45 To 51 9423389183 02182- [email protected] 220516 m 251 KARMALA 8 43 Madha 244 Karmala D R Tijore Asst. Register 52 To 55, 130 9011541697 [email protected] Karmala To 133 om 252 KARMALA 9 43 Madha 244 Karmala M. S. Kambale Nagar Parishad 56 To 60, 9404318482 02182- [email protected] Karmala 125,126,128, 222033 om 129 253 KARMALA 10 43 Madha 244 Karmala A A Jarande O S P S Karmla , P S 61 To 64, 9421263018 02182- [email protected] Karmala 121,122,124, 220315 m 127 254 KARMALA 11 43 Madha -

After Corrections Electoral Rolls of Collegium of HEADS of the DEPARTMENTS for the Election to the BOARDS of STUDIES Coming Under The
SOLAPUR UNIVERSITY, SOLAPUR After Corrections Electoral Rolls of Collegium of HEADS OF THE DEPARTMENTS for the Election to the BOARDS OF STUDIES coming under the FACULTY OF COMMERCE AND MANAGEMENT [Section 40 (2) (c) of the M. P. U. Act, 2016] Published on 19th August, 2017 SOLAPUR UNIVERSITY, SOLAPUR After Corrections Electoral Roll of Collegium of Heads of the Departments in affiliated colleges / recognised institutions for the Election to the BOARD OF STUDIES in COMMERCE Published on 19 th August, 2017 Centre No. 1 :- Sangameshwar College, Solapur 1 Kamble Dnyanoba Sidram M Sangmeshwar College, Sat Rasta, Solapur. 2 Varade Vaishali Keshav F V. G. Shivdare College of Arts & Science, Solapur. Centre No. 2 :- D.B.F. Dayanand College of Arts & Science, Solapur. 4 Yajamanya Swarnalata Raghav F D. A. V. Velankar College of Commerce, Solapur. Centre No. 3 :- Walchand College of Arts & Science, Solapur. 5 Bansude Ranjana Vasant F A. R. Burla Mahila Varistha Mahavidyalaya, Solapur. 6 Kamble Babruvhan Namdev M Hirachand Nemachand College of Commerce, Solapur After Corrections Final Electoral Roll HOD 2017.xls M = Male, F= Female 1 / 9 Centre No. 4 :- C. B. Khedagi's Basweshwar, Raja Vijaysinha Commerce, Raja Jaysinha Arts College, Akkalkot, Tal. Akkalkot, Dist. Solapur. 7 Wale S. S. M C. B. Khedgi's Basaveshwar College, Akkalkot, Tal. Akkalkot, Dist. Solapur. Centre No. 7 :- Yashwantrao Chavan Mahavidyalaya, Karmala, Tal. Karmala, Dist. Solapur. 8 Khuntale Vijaykumar Purushottam M Yashwanthrao Chavan Mahavidyalaya Karmala, Tal. Karmala, Dist. Solapur Centre No. 8 :- Shankarrao Mohite Mahavidyalaya, Akluj, Tal. Malshiras, Dist. Solapur 9 Gaikwad Ratnadeep Laxman M Shankarrao Mohite Mahavidyalaya, Akluj, Tal. -

Form-1 Stone Quarry Ministry of Environment & Forests
APPLICATION FOR ENVIRONMENTAL CLEARANCE Vide para 6 of S. O. 1533 dated 14 September 2006 FORM-1 OF STONE QUARRY Survey No. -40/1/1/A, Area- 1.31 Ha Village- Wagholiwadi, Tehsil- Mohol, Dist-Solapur Submitted to MINISTRY OF ENVIRONMENT & FORESTS STATE LEVEL EXPERT APPRAISAL COMMITTEE (SEAC Maharashtra) * Project Proponent Sitaram Jalindar Lande At post Wagholiwadi, Tehsil- Mohol, Dist-Solapur * EIA Consultant SRUSHTI SEWA Harihar Apartment,Agresen Road, Near Adivasi Bhavan , Giripeth, Nagpur-440010(Maharashtra) Phone : 0712-688545 Email : [email protected] Accredited by NABET: No.- NABET/EIA/127/SA062/0613 Dated 26/06/2013 April-2016 Form-1 REVISED FORM – 1 APPLICATION FOR OBTAINING ENVIRONMENTAL CLEARANCE VIDE PARA 6 OF S.O.1533 OF MOEF NOTIFICATION DATED 14.09. 2006 (I) Basic Information: S. Item Details No 1. Name of the Project/s : STONE QUARRY 2. S. No. in the schedule : 1 (a) as per S.O.1533dt 14.09.2006 3. Propo sed Capacity / area / length : 1.31 Ha / tonnage to be 2100 Brass/Month Stone handled/command area/lease area/ number of wells to be drilled. 4. New/Expansion/Modernization : Existing lease applied for EC Vide OM No. L - 11011/47/2011-IA.II(M) dated 18.05.12 5. Existing Capacity/Area etc. : 1.31 Ha Existing capacity-2400 Brass/Month Stone 6. Category of project i.e. ‘A’ or ‘B’ : ‘B’ 7. Does it attract the general : No condition? If yes, please specify. 8. Does it attract the specific : No condition? If yes, please specify. 9. Location : Location Map 1 Plot/Survey/Khasra No. -

Village Map Taluka: Malshiras District: Solapur Madha
Karmala Baramati Village Map Taluka: Malshiras District: Solapur Madha Kurbavi Indapur Deshmukhwadi Ekshiv Shindewadi Tambewadi Kalamboli Dombalwadi Hanumanwadi Bangarde Palasmandal Pirale Phaltan Dharmpuri µ Dahigaon 4.5 2.25 0 4.5 9 13.5 Gursale Motewadi Sangam Babhulgaon km Kadamwadi Wafegaon Umbare Dahigaon Ganeshgaon Fondshiras Pratapnagar (N.V.) Tirwandi Tambave Morochi Chakore Bijvadi Wagholi Markadwadi Kondabavi Anandnagar Location Index Karunde Natepute (CT) Tamsidwadi Savatgavhan Lawang Medad Kacharewadi Sangramnagar (N.V.) (CT) Akluj (CT) Bagechiwadi Vatpali (N.V.) District Index Malinagar Pimpari Nandurbar Purandawade Bhandara Yashawantnagar Mahalung Mandave Sadashivnagar Yeliv MALSHIRAS Girzani Malewadi Umbare (Velapur) Dhule Amravati Nagpur Gondiya !(Malshiras Jalgaon Kothale Mire Akola Wardha Buldana Shiwarvasti (N.V.) Paniv Chaundeshwarwadi Kondarpatta Nashik Washim Chandrapur Lonand Yavatmal Jadhavwadi Neware Dhulenagar N.V.) Vijaywadi Palghar Aurangabad Londhe Mohitewadi Jalna Gadchiroli Khandali Hingoli Bhamburdi Borgaon Thane Dattanagar Ahmednagar Parbhani Fadtari Dombalwadi Vizori Malewadi Mumbai Suburban Nanded Jambud Mumbai Bid Giravi Motewadi Pune Raigarh Bidar Kanher Khudus Latur Malkhambi Osmanabad Islampur Ughadewadi Nitavewadi (N.V.) Vithalwadi Solapur Zunjewadi Pisewadi Satara Ratnagiri Bhamb Sangli Velapur Bondale Khalawe Maharashtra State Rede Mandaki Goradwadi Kolhapur Tarangfal Sindhudurg Nimgaon Dharwad Dasur Tondale Dhanore Jalbhavi Taluka Index Shendechinch Chandapuri Magarwadi (N.V.) Pandharpur Karmala -

Ratio of Agriculture in Drought Prone Area and Indexing of Irrigationin Micro Area in Solapur District
Aayushi International Interdisciplinary Research Journal (AIIRJ) Vol - VI Issue - III MARCH 2019 Peer Review e-Journal Impact Factor 5.707 ISSN 2349-638x Ratio of Agriculture in Drought Prone Area And Indexing of Irrigationin Micro Area in Solapur District Mr. A. G. Nimase Assistant Professor Department of Geography Chhatrapati Shivaji College, Satara Abstract Drought is the basicevent in the natural climatic system. When drought occurs there is an acute scarcity of water over the land surface. In extreme conditions the water-table is so-lowered that the wells/pumps sets are failed to discharge the water, as result not only the crops are suffered but the human being also face a crucial problem for drinking water.There is no operational practice to forecast the draught. One of the suggestions is to update mapping of draught prone areas developmental planning.Statistical analysis is very important in such planning. The main aim of this research paper is, to analyze, region wise relative requirement of water foragriculture at village level. Regionalization of study area has been done on the basis of decadal population growth and density of population per square km. Thus, ultimately evolved the nine micro regions. Further it work out that “Index of Irrigation Requirement”(Ir.). There are 104 villages included in Mohol tahsil. Out of them 47 villages having very low (Ir. <0.15) requirement of water for agriculture, whereas 29 villages shows low (Ir. 0.15 – 0.30), 11 denotes medium (Ir. 0.30 – 0.45), 18 are high (Ir. 0.45 – 0.60) and 9 villages observed very high ( Ir. -
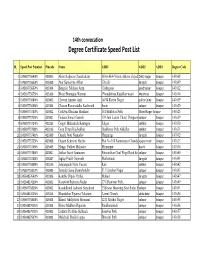
Speed Post All List.Xlsx
14th convocation Degree Certificate Speed Post List SL Speed Post Number Pincode Name ADD1 ADD2 ADD3 Degree Code 1 EM569775640IN 413005 Akim Kalpana Chandrakant H.No.46/4 Vinoba Bhave ZopadpDatta nagar Solapur 143608 2 EM569775653IN 413309 Atar Sumaiyya Akbar Gherdi, Sangola Solapur 143609 3 EM569775667IN 413304 Bengale Malhari Arun Gadegaon pandharpur Solapur 143612 4 EM569775675IN 413109 Bhise Bhanudas Waman Phondshiras,Kadalkar wasti Malshiras Solapur 143614 5 EM569775684IN 413005 Chavan Amruta Anil 14/54 Kavita Nagar police Colny Solapur 143619 6 EM569775698IN 413304 Chavan Revansiddha Kashinath Suste solapur Solapur 143620 7 EM569775707IN 413002 Gaikwad Reshma Shrikant 181 Budhwar Peth Milind Nagar Solapur 143625 8 EM569775715IN 413001 Godase Sonali Ganesh 159 Juni Laxmi Chawl Dongaon solapur Solapur 143629 9 EM569775724IN 413225 Gogav Mahantesh Rautappa Salgar aaklkot Solapur 143630 10 EM569775738IN 413216 Gore Shruti Rajshekhar Budhavar Peth Akkalko aaklkot Solapur 143631 11 EM569775741IN 413309 Gujale Jyoti Namadev Hangirage Sangola Solapur 143632 12 EM569775755IN 413008 Gujare Sukeshni Hariba Plot No 168 Ramnaryan Chandakvijapur road Solapur 143633 13 EM569775769IN 413403 Hange Pallavi Mahadev Mirzanpur Barshi Solapur 143634 14 EM569775786IN 413001 Jadhav Swati Santaram Patwardhan Chal Wagi Road Solsolapur Solapur 143640 15 EM569775790IN 413307 Jagtap Pratik Gopinath Methawade Sangola Solapur 143641 16 EM569775809IN 413216 Jainjangade Nitin Vasant Kini aaklkot Solapur 143642 17 EM569775812IN 413003 Jamadar Sana Shamshoddin 51/1 -

Brief Industrial Profile of Solapur District
MSME-DI Mumbai Brief Industrial Profile of Solapur District MSME – Development Institute, Ministry of MSME, Govt. of India, Kurla Andheri Road. Saki Naka, Mumbai 400 072 Tel. No: 022-28576090/3091/7166 Fax: 022-2857 8092 Email: [email protected] Website: www.msmedimumbai.gov.in 1 Contents S.No. Topic Page No. 1. General Characteristics of the District 3 1.1 Location & Geographical Area 3 1.2 Topography 3 1.3 Availability of Minerals 4 1.4 Forest 5 1.5 Administrative set up 6 2 District at a Glance 6 2.1 Existing status of Industrial area in the district of 8 Solapur 3 Industrial Scenario of Solapur 9 3.1 Industries at a Glance 9 3.2 Year wise trend 9 3.3 Details of existing Micro & Small Enterprises & 10 Artisan units in the district 3.4 Large Scale Industries/Public Sector undertakings 12 3.5 Major Exportable items 14 3.6 Growth Trend 14 3.7 Vendorisation / Ancillarisation of the Industry 14 3.8 Medium scale Enterprises 15 3.9 Service Enterprises 15 3.9.2 Potential areas for service Industry 16 3.10 Potential for new MSMEs 16 4 Existing Clusters of Micro & Small Enterprise 18 4.1 Detail of major cluster 18 4.1.1 Manufacturing sector 18 4.2.1 Cotton Terry Towels,chanddars and Grey Fabrics, 18 Solapur 5. General issues raised by Industries Association 19 6. Steps to set up MSMEs 20 2 Brief Industrial Profile of Solapur District 1) General Characteristics of the District & Brief History: The Solapur District was ruled by various dynasties such as Andhrabhratyas, Chalukyas, Rashtrkutas, Yadavas and Bahamanis.