FORMULATION and EVALUATION of MEDICATED CANDY CONTAINING ALBENDAZOLE for PEDIATRIC Punam V
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Edible Glass
Edible Glass Edible Glass By Josh Pomeroy Abstract Students learn the principles of edible glass by making a supersaturated sugar solution. Equipment 1. Hot plate 2. Heavy pot with lid 3. Water 4. Thermometer 5. Watch or Clock with seconds place 6. Balance or scale 7. Oven mitts 8. Hot pad 9. 2 cups sugar 10. ¾ cup light corn syrup 11. 1 tablespoon unsalted butter 12. Plate 13. Long wooden spoon 14. Drinking Glass 15. Food Coloring 16. Flavoring 17. Cookie Sheet greased with vegetable oil 18. Sturdy Toothpicks 19. Aluminum Foil Grade Level This activity is suitable for Late Elementary, Middle and High School Students. State Standards Met. Standard 1 – Analysis, Inquiry, and Design Standard 4 – Physical Setting and Living Environment Standard 7 – Interdisciplinary Problem Solving Introduction The goal of the following lab is to provide an interesting experiment which students of many different levels can perform, and to provide information to present the experiment from several different perspectives. The lab will begin with an introduction to common sugars and will include some discussion of temperature and thermometry, some basic thermodynamics, and the experimental procedure to make the candy glass. Finally I have provided some sample questions to encourage thought and to further develop an understanding of how one uses measurements to determine science. Sugars – From Glucose to Cellulose Glucose? Cellulose? Huh? You may never have heard of these names before, but they are all around you and in the food you eat. Glucose is the simplest type of sugar, the fuel your cells burn to give you energy. -

Confections of a Victorian Lady
C torian Lady o By Suszanne Perfect Originally published in 1982 by New nf England Press Neither this book nor any parts within may be reproduced in any form, stored ec in any format, or transmitted in any way, or by any means—electronic, mechanical, photocopy, recording, or ti otherwise—without the prior written permission of the publisher except as provided by United States copyright o law. Published on behalf of The Governor’s ns Mansion, LLC by bent spoon Media, LLC 19595 Waterford Place, Shorewood, MN, 55331 of [email protected] Phone: (925) 588-1983 a www.thegovernorsmansion.org V ic Contents Introduction .............................................................. 5 FRUIT ...................................................................... 8 NUTS ...................................................................... 13 TAFFY & FONDANT............................................ 18 Caramels and Creams ............................................. 27 FUDGES ................................................................ 32 Misc ........................................................................ 37 INDEX .................................................................... 47 Introduction FRUIT Candied Apricots Soak a pound of apricots for 2–3 hours, no longer. Then drain thoroughly. Take 2 cups of sugar and a cup of water and boil until it threads. Add apricots and cook slowly until almost all the juice is absorbed. Remove from fire, shape and roll in granulated sugar. Candied Cherries Use the best Morellos for this purpose. Weigh the cherries, stone them, and sprinkle half their weight in sugar. Let stand overnight in the ice chest. In the morning remove the cherries, pour off all liquid into preserving kettle. Let boil for half an hour. Divide the fruit into small amounts and cook each amount in syrup for 20 minutes. Remove all fruit from syrup and cook it until it is thick and ropy. Spread cherries on baking sheets, cover with cheesecloth, and place in the sun. -

You Can Eat Crystal and Glass??
You Can Eat Crystal and Glass?? The same, but different: These two lollipops look and feel very different. Why are they so different if they’re made out of the same stuff - sugar? It has to do with how they are made. The rock candy on the right slowly formed crystals from sugar dissolved in water. The sugar molecules are in an organized pattern. The lollipop on the left was made from sugar solution heated to about 300 °F. It cooled so quickly from the liquid that there was no time for crystals to form, and the sugar molecules are all jumbled up. A solid material without crystals is called amorphous, or glass, and one with crystals is called crystalline. Which lollipop is glass, and which is crystalline? In these activities, you will explore the differences between amorphous and crystalline materials by making edible glass and crystals. Activity: Edible Glass Time: 45 minutes. Parents, please supervise children. Materials: • 1 ½ cups sugar • Saucepan • ¾ cup water • Large wooden or plastic spoon • ½ cup light corn syrup • Measuring cup • 1 tsp. extract or oil flavoring • Candy thermometer • Food coloring • Baking sheet • Confectioner’s sugar What to do: • Spread a layer of confectioner’s sugar onto the baking sheet. • Mix water, sugar and corn syrup in the saucepan on medium heat until the sugar is dissolved. • Boil on medium heat, checking the temperature every so often until the mixture reaches 300 °F. This will take several minutes. Be very careful with the hot sugar solution. • Remove from heat. Mix in flavoring and food coloring. • Pour onto the baking sheet. -

Syrup and Candy Recipes
Sugar and Candy Recipes for Feeding Bees Feeding bees can quickly become confusing, especially for the new beekeeper. This chart will help you easily mix the desired quantity and ratio of syrup. Keep in mind that you will end up with slightly more syrup than the amount of water used (fortunately you can place any extra in the fridge for a few days). Enjoy! Sugar Syrup Sugar Candy To make: Heat desired amount of water until This is the preferred food for supplementary almost boiling. Do not boil water. Remove from feeding beginning in late February - early March. heat and stir in sugar. Allow to cool and feed to It is concentrated and has its own water supply. bees. Boiling water will result in a crystallized This is one time where you DO boil the sugar and solution. water together. Bring 2 cups water and 5 pounds (10 cups) of 1:1 Syrup white granulated sugar to a boil at 234ºF (soft 1:1, or One-to-One syrup can be used for ball stage) and cook for 30 seconds. supplemental spring feeding and to encourage the Cool to 180ºF. (putting pot into sink full of drawing of comb. water will speed this up!) 1-part (by weight) sugar 1-part (by weight) water Then stir vigorously. Pour into a mold made of cardboard or a 1:1 sugar syrup (used in Spring) container lined with waxed paper or butcher’s 4 cups of sugar to 4 cups (2 pints) water paper. The candy will harden as it cools in 2-3 10 cups of sugar to 10 cups (5 pints) water hours. -

(12) Patent Application Publication (10) Pub. No.: US 2009/0004248 A1 Bunick Et Al
US 20090004248A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2009/0004248 A1 Bunick et al. (43) Pub. Date: Jan. 1, 2009 (54) DUAL PORTION DOSAGE LOZENGE FORM Related U.S. Application Data (60) Provisional application No. 60/947,004, filed on Jun. (76) Inventors: Frank Bunick, Randolph, NJ (US); 29, 2007. Joseph Luber, Quakertown, PA Publication Classification (US); Stephan G. Wiet, Morristown, NJ (US); Gerard P. (51) Int. Cl. McNally, Berwyn, PA (US); David A69/20 (2006.01) Wynn, Huntingdon Valley, PA (US) A6IR 9/28 (2006.01) A63L/485 (2006.01) (52) U.S. Cl. ......................................... 424/440: 514/289 Correspondence Address: PHILIP S. JOHNSON (57) ABSTRACT JOHNSON & JOHNSON The present invention relates to a dosage form including both ONE JOHNSON & JOHNSON PLAZA a disintegrative tablet portion and a hard candy portion, NEW BRUNSWICK, NJ 08933-7003 (US) wherein: (i) the disintegrative tablet portion comprises at least one pharmaceutically active agent, and (ii) the hard candy portion covers at least 20% of the surface of the disintegrative (21) Appl. No.: 12/143,916 tablet portion, and wherein the disintegration time of the hard candy portion is at least ten times longer than the disintegra (22) Filed: Jun. 23, 2008 tion time of the disintegrative tablet portion. US 2009/0004248 A1 Jan. 1, 2009 DUAL PORTION DOSAGE LOZENGE FORM rated by reference. As used herein, all percentages are by weight unless otherwise specified. CROSS REFERENCE TO RELATED APPLICATION Disintegrative Tablet Portion 0008. The dosage form of the present invention includes a 0001. This application claims priority to prior U.S. -

Cornell Maple Bulletin 208: Molded Sugar Candy
Cornell Maple Bulletin 208 (2007) Molded Sugar Candy (Soft Sugar Candy) by STEPHEN CHILDS Adapted from C.O. Willits and C.H. Hill 1976. Maple Syrup Producers Manual. USDA Agriculture Handbook No. 134 and North American Maple Syrup Producers Manual, 2nd ed, 2006 Background Molded sugar candy is a popular maple value-added product. Approximately 7 ½ pounds (3.4 Kg) of molded sugar can be prepared from one gallon (4.4 liters) of maple syrup. Molded maple sugar contains nothing other than maple sugar with little or no free syrup, thus it is stiff and can be molded into a variety of shapes. The crystals in molded sugar are larger than in maple cream and can be sensed on the tongue, but they should not be so large as to have an unpleasant sandy or gritty texture. The temperature and humidity of the room where you make candy will affect the quality of the candy and repeatability of the process. Best results will occur at normal room temperature (68-72oF) and humidity in the 40-45% range. Dry days are best and rainy or humid days should be avoided. Syrup Grade Syrup Grade and Invert Sugar and Invert Molded sugar can be made from any of the top three grades of syrup with an invert sugar Sugar level between 0.5% and 1.5% with the ideal being about 1%. Lower levels of invert sugar in the syrup tend to produce large crystals that give the sugar a grainy texture. Lower levels also can cause problems because the syrup is more likely to crystallize in the pan as it is cooling before stirring. -
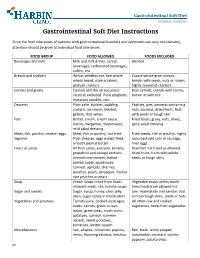
Gastrointestinal Soft Diet Instructions
Gastrointestinal Soft Diet GENERAL SURGERY Gastrointestinal Soft Diet Instructions Since the food tolerances of patients with gastrointestinal disorders and symptoms can vary considerably, attention should be given to individual food tolerances. FOOD GROUP FOOD ALLOWED FOODS EXCLUDED Beverages and milk Milk and milk drinks, cereal Alcohol beverages, carbonated beverages, coffee, tea Breads and crackers White, seedless rye, fine whole- Coarse whole-grain breads, wheat bread, plain crackers, breads with seeds, nuts or raisins, graham crackers highly seasoned crackers Cereals and grains Cooked and dry cereal unless Bran cereals, cereals with raisins, listed as excluded. Plain spaghetti, brown or wild rice macaroni noodles, rice. Desserts Plain cake, cookies, pudding, Pastries, pies, desserts containing custard, ice cream, sherbet, nuts, coconut, dried fruits, fruit gelatin, fruit whips with seeds or tough skin Fats Butter, cream, cream sauce, Fried foods, gravy, nuts, olives, bacon, margarine, mayonnaise, spicy salad dressing mild salad dressing Meat, fish, poultry, cheese, eggs, Meat, fish or poultry, not fried. Fried meats, fish or poultry, highly legumes Plain cheeses, eggs except fried, seasoned cold cuts or sausage, smooth peanut butter fried eggs Fruits or juices All fruit juices, avocado, banana, Raw fruit not listed as allowed, grapefruit and orange sections dried fruits, fruits with edible without membranes, baked seeds or tough skins peeled apple; applesauce. Canned: apricots, cherries, peaches, pears, pineapple. Peeled ripe peaches -
WO 2016/102186 Al 30 June 2016 (30.06.2016) P O P C T
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date WO 2016/102186 Al 30 June 2016 (30.06.2016) P O P C T (51) International Patent Classification: (81) Designated States (unless otherwise indicated, for every A23G 3/34 (2006.01) A23G 3/56 (2006.01) kind of national protection available): AE, AG, AL, AM, A23G 3/54 (2006.01) AO, AT, AU, AZ, BA, BB, BG, BH, BN, BR, BW, BY, BZ, CA, CH, CL, CN, CO, CR, CU, CZ, DE, DK, DM, (21) Number: International Application DO, DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, PCT/EP20 15/079073 HN, HR, HU, ID, IL, IN, IR, IS, JP, KE, KG, KN, KP, KR, (22) International Filing Date: KZ, LA, LC, LK, LR, LS, LU, LY, MA, MD, ME, MG, ' December 2015 (09.12.2015) MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, NZ, OM, PA, PE, PG, PH, PL, PT, QA, RO, RS, RU, RW, SA, SC, (25) Filing Language: English SD, SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, TM, TN, (26) Publication Language: English TR, TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, ZW. (30) Priority Data: (84) Designated States (unless otherwise indicated, for every MI2014A002246 23 December 2014 (23. 12.2014) IT kind of regional protection available): ARIPO (BW, GH, GM, KE, LR, LS, MW, MZ, NA, RW, SD, SL, ST, SZ, (71) Applicant: PERFETTI VAN MELLE SPA [ΓΓ/ΙΤ]; Via TZ, UG, ZM, ZW), Eurasian (AM, AZ, BY, KG, KZ, RU, XXV Aprile, 7, 20020 Lainate (MI) (ΓΓ). -

Trade Mark Ex Parte Decision O/204/18
o/204/18 TRADE MARKS ACT 1994 IN THE MATTER OF APPLICATION NUMBER 3250658 BY HELEN ROSEMARY BROWN TO REGISTER THE FOLLOWING TRADE MARK IN CLASS 30: mouth wateringly special 1 o/204/18 TRADE MARKS ACT 1994 IN THE MATTER OF APPLICATION NUMBER 3250658 BY HELEN ROSEMARY BROWN TO REGISTER THE FOLLOWING TRADE MARK IN CLASS 30: mouth wateringly special Background 1. On 16 August 2017 Helen Rosemary Brown (‘the applicant’) applied to register the above mark for goods in class 30 as listed in Annex A of this decision. 2. On 25 August 2017, the Intellectual Property Office (‘IPO’) issued an examination report in response to the application. In that report, an objection was raised under section 3(1)(b) of the Trade Marks Act 1994 (‘the Act’) which read as follows: “The application is not acceptable in Class 30. There is an objection under Section 3(1)(b) of the Act as the mark is devoid of any distinctive character. This is because the expression ‘mouth wateringly special’ merely serves a promotional and laudatory function e.g. the average consumer would perceive the expression ‘mouth wateringly special’ as a laudatory message with the purpose of highlighting a positive attribute of the goods being offered. It is considered that the average consumer, when greeted with the sign ‘mouth wateringly special’ would not attribute any trade mark significance to the expression and would instead merely perceive the sign as a promotional intimation that the food products provided by the applicant are better than the average product and have an incredible taste. -
Candy Making
CANDY MAKING IN THE HOME CHRISTINE TERHUNE HERRICK “ & “ - A u thor of The Little Dinner, The Chafing D ish ” “ Sa er First A id the You n Hou s k r wy , to g e eepe . “ & “ Su nda N i ht Su rs and Like y g ppe . Mother Used to M I RAND McNA£L§f COMPANY r &mac 3 NEW You ; OONTENTS COUNSEL ON CANDY MAKING BUm nsco'rcn AND CAEAN EL s ‘ A N IEs 2 COCONUT, CEE N , A D MAPL E CAND 9 FUDcEs AND SIMPLE NUT CANDIEs Tm . POPCORN, AND CANDY Dnops Nm AND FRUIT CANDIE s AND MAESEN ALLOWs UG R ND R A E MOL ASSES, S A , A C EAM C NDI s 68 PlENcn CANDIEs AND FONDANT CHOCOL ATES, NUTS, PEUrrs WITH COOKED FONDANT MISCELL ANEOUS SWEE 'I NE A'rs w rrE COOKED FONDANT ONEONs CO FECTIO S B , N N , AND DIPPED CAND IE s HOMEMADE COUGE CANDIE s CA NDY MA KING COUNSEL ON CANDY MAKING The American people have been called the most excessive can dy eaters in the w orld ; an d the nationalfond ness for sw eets has been termed a rim r can no ou a c e . The e be d bt th t in m any cases the habit approaches il n r per ou sly ear dange . Yet there is a degree of reason in c i ar is f - the pra t ce . Su g a orce maker ; Americans are conceded to be excep tionally energetic and restless ; the ou tpu t of nerve and mu scle strength m c an l n an u st be onst t y re ew ed, d the ’ craving for sw eets is one of Natu re s e orts r r as fi to epai w te . -

Sweet Treats Around the World This Page Intentionally Left Blank
www.ebook777.com Sweet Treats around the World This page intentionally left blank www.ebook777.com Sweet Treats around the World An Encyclopedia of Food and Culture Timothy G. Roufs and Kathleen Smyth Roufs Copyright 2014 by ABC-CLIO, LLC All rights reserved. No part of this publication may be reproduced, stored in a retrieval system, or transmitted, in any form or by any means, electronic, mechanical, photocopying, recording, or otherwise, except for the inclusion of brief quotations in a review, without prior permission in writing from the publisher. The publisher has done its best to make sure the instructions and/or recipes in this book are correct. However, users should apply judgment and experience when preparing recipes, especially parents and teachers working with young people. The publisher accepts no responsibility for the outcome of any recipe included in this volume and assumes no liability for, and is released by readers from, any injury or damage resulting from the strict adherence to, or deviation from, the directions and/or recipes herein. The publisher is not responsible for any readerÊs specific health or allergy needs that may require medical supervision or for any adverse reactions to the recipes contained in this book. All yields are approximations. Library of Congress Cataloging-in-Publication Data Roufs, Timothy G. Sweet treats around the world : an encyclopedia of food and culture / Timothy G. Roufs and Kathleen Smyth Roufs. pages cm Includes bibliographical references and index. ISBN 978-1-61069-220-5 (hard copy : alk. paper) · ISBN 978-1-61069-221-2 (ebook) 1. Food·Encyclopedias. -

Trade Marks Appointed Person Decision O/452/18
O-452-18 TRADE MARKS ACT 1994 IN THE MATTER OF APPLICATIONS Nos. 3250658 AND 3250660 IN THE NAME OF HELEN ROSEMARY BROWN AND IN THE MATTER OF APPEALS BY HELEN ROSEMARY BROWN AGAINST DECISIONS OF MS LINDA SMITH DATED 29 MARCH 2018 _______________ DECISION _______________ Introduction 1. On 16 August 2017, Helen Rosemary Brown (“the Applicant”) made 2 x applications to register the designation MOUTH WATERINGLY SPECIAL for use as a trade mark in the UK as follows: (1) Application number 3250658 in Class 30 for the goods listed at Annex A to this decision; (2) Application number 3250660 in Class 29 for the goods listed at Annex B to this decision. 2. The Registrar raised objections to the Applications under Section 3(1)(b) of the Trade Marks Act 1994 which states: 3. - (1) The following shall not be registered – […] (b) trade marks which are devoid of any distinctive character …” 3. The accepted legal approach to Section 3(1)(b) was summarised by the Court of Justice of the European Union (“CJEU”) (in relation to the equivalent art. 7(1)(b) of Regulation (EU) 2017/1001 on the EU trade mark) in Case C-265/09 P, OHIM v. BORCO-Marken-Import Matthiesen GmbH & Co. KG. [2010] ECR I-8265 at paragraphs 30 – 37 and 45 (without authorities): “30. Under that provision [art. 7(1)(b)/s. 3(1)(b)], marks which are devoid of any distinctive character are not to be registered. 31. According to settled case‑law , for a trade m ark to possess distinctive character for the purposes of that provision, it must serve to identify the product in respect of which registration is applied for as originating from a 1 O-452-18 particular undertaking, and thus to distinguish that product from those of other undertakings … 32.