Umbilical Cord Prolapse (Green-Top Guideline No
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
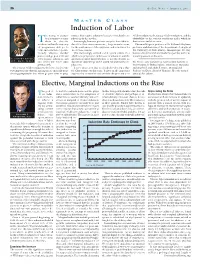
Induction of Labor
36 O B .GYN. NEWS • January 1, 2007 M ASTER C LASS Induction of Labor he timing of parturi- nancies that require induction because of medical com- of labor induction, the timing of labor induction, and the tion remains a conun- plications in the mother. advisability of the various conditions under which in- Tdrum in obstetric Increasingly, however, patients are apt to have labor in- duction can and does occur. medicine in that the majority duced for their own convenience, for personal reasons, This month’s guest professor is Dr. William F. Rayburn, of pregnancies will go to for the convenience of the physician, and sometimes for professor and chairman of the department of ob.gyn. at term and enter labor sponta- all of these reasons. the University of New Mexico, Albuquerque. Dr. Ray- neously, whereas another This increasingly utilized social option ushers in a burn is a maternal and fetal medicine specialist with a na- portion will go post term and whole new perspective on the issue of induction, and the tional reputation in this area. E. ALBERT REECE, often require induction, and question is raised about whether or not the elective in- M.D., PH.D., M.B.A. still others will enter labor duction of labor brings with it added risk and more com- DR. REECE, who specializes in maternal-fetal medicine, is prematurely. plications. Vice President for Medical Affairs, University of Maryland, The concept of labor induction, therefore, has become It is for this reason that we decided to develop a Mas- and the John Z. -

Association Between First Caesarean Delivery and Adverse Outcomes In
Hu et al. BMC Pregnancy and Childbirth (2018) 18:273 https://doi.org/10.1186/s12884-018-1895-x RESEARCHARTICLE Open Access Association between first caesarean delivery and adverse outcomes in subsequent pregnancy: a retrospective cohort study Hong-Tao Hu1†, Jing-Jing Xu1†, Jing Lin1, Cheng Li1, Yan-Ting Wu2, Jian-Zhong Sheng3, Xin-Mei Liu4,5* and He-Feng Huang2,4,5* Abstract Background: Few studies have explored the association between a previous caesarean section (CS) and adverse perinatal outcomes in a subsequent pregnancy, especially in women who underwent a non-indicated CS in their first delivery. We designed this study to compare the perinatal outcomes of a subsequent pregnancy in women who underwent spontaneous vaginal delivery (SVD) or CS in their first delivery. Methods: This retrospective cohort study included women who underwent singleton deliveries at the International Peace Maternity and Child Health Hospital from January 2013 to December 2016. Data on the perinatal outcomes of all the women were extracted from the medical records. Multivariate logistic regression was conducted to assessed the association between CS in the first delivery and adverse perinatal outcomes in the subsequent pregnancy. Results: CS delivery in the subsequent pregnancy was more likely for women who underwent CS in their first birth than for women with previous SVD (97.3% versus 13.2%). CS in the first birth was also associated with a significantly increased risk of adverse outcomes in the subsequent pregnancy, especially in women who underwent a non- indicated CS. Adverse perinatal outcomes included pregnancy-induced hypertension [adjusted odds ratio (OR), 95% confidence interval (CI): 2.20, 1.59–3.05], gestational diabetes mellitus (1.82, 1.57–2.11), gestational anaemia (1.27, 1. -

ABCDE Acronym Blood Transfusion 231 Major Trauma 234 Maternal
Cambridge University Press 978-0-521-26827-1 - Obstetric and Intrapartum Emergencies: A Practical Guide to Management Edwin Chandraharan and Sir Sabaratnam Arulkumaran Index More information Index ABCDE acronym albumin, blood plasma levels 7 arterial blood gas (ABG) 188 blood transfusion 231 allergic anaphylaxis 229 arterio-venous occlusions 166–167 major trauma 234 maternal collapse 12, 130–131 amiadarone, overdose 178 aspiration 10, 246 newborn infant 241 amniocentesis 234 aspirin 26, 180–181 resuscitation 127–131 amniotic fluid embolism 48–51 assisted reproduction 93 abdomen caesarean section 257 asthma 4, 150, 151, 152, 185 examination after trauma 234 massive haemorrhage 33 pain in pregnancy 154–160, 161 maternal collapse 10, 13, 128 atracurium, drug reactions 231 accreta, placenta 250, 252, 255 anaemia, physiological 1, 7 atrial fibrillation 205 ACE inhibitors, overdose 178 anaerobic metabolism 242 automated external defibrillator (AED) 12 acid–base analysis 104 anaesthesia. See general anaesthesia awareness under anaesthesia 215, 217 acidosis 94, 180–181, 186, 242 anal incontinence 138–139 ACTH levels 210 analgesia 11, 100, 218 barbiturates, overdose 178 activated charcoal 177, 180–181 anaphylaxis 11, 227–228, 229–231 behaviour/beliefs, psychiatric activated partial thromboplastin time antacid prophylaxis 217 emergencies 172 (APTT) 19, 21 antenatal screening, DVT 16 benign intracranial hypertension 166 activated protein C 46 antepartum haemorrhage 33, 93–94. benzodiazepines, overdose 178 Addison’s disease 208–209 See also massive -

Managing the Risk of Uterine Rupture During a Trial of Labor After Cesarean Section
Managing the Risk of Uterine Rupture During a Trial of Labor After Cesarean Section By NORCAL Mutual Insurance Company Introduction While a successful vaginal birth after cesarean section (VBAC) is associated with less morbidity and mortality than repeat cesarean section (C-section), an unsuccessful VBAC is associated with a small but significant risk of uterine rupture that can result in death or serious injury to both the mother and the infant.1 When a trial of labor after C-section (TOLAC) ends in uterine rupture, emergency C-section, and the delivery of an infant with brain injuries, there is a good chance that the child’s This article originally appeared in the parents will file a lawsuit, or at least September 2011 issue of Claims Rx. It consider it. It should be noted that has been edited by Drs. Mark Zakowski, a plaintiff’s attorney is supposed to Patricia Dailey and Stephen Jackson prove duty (responsibility of the to meet the educational needs of physicians involved), negligence anesthesiologists, and is reprinted, as (care provided was below the changed, with permission. ©Copyright standard of care) and causation 2011, NORCAL Mutual Insurance Co. (negligence led to the injury) All Rights Reserved. Reproduction as well as injury. However, the permissible with written permission plaintiffs probably won’t focus on and credit. whether the standard of care was met, and their attorney might not either. In these types of cases, the degree of the infant’s brain injuries tends to over- shadow other liability issues. This can carry through to trial because juries are generally biased toward severely brain-injured infants and the parents who must provide for them. -

Umbilical Cord Prolapse Guideline
Umbilical Cord Prolapse Guideline Document Control Title Umbilical Cord Prolapse Guideline Author Author’s job title Specialty Trainee in Obstetrics and Gynaecology Directorate Department Women’s and Children’s Obstetrics and Gynaecology Date Version Status Comment / Changes / Approval Issued 1.0 Mar Final Approved by the Maternity Services Guideline Group in 2011 April 2011. 1.1 Aug Revision Minor amendments by Corporate Governance to 2012 document control report, headers and footers, new table of contents, formatting for document map navigation. 2.0 Feb Final Approved by the Maternity Services Guideline Group in 2016 February 2016. 2.1 Apr Revision Harmonised with Royal Devon & Exeter guideline 2019 3.0 May Final Approved by Maternity Specialist Governance Forum 2019 meeting on 01.05.2019 Main Contact ST1 O&G Tel: Direct Dial– 01271 311806 North Devon District Hospital Raleigh Park Barnstaple Devon EX31 4JB Lead Director Medical Director Superseded Documents Nil Issue Date Review Date Review Cycle May 2019 May 2022 Three years Consulted with the following stakeholders: (list all) Senior obstetricians Senior midwives Senior management team Filename Umbilical Cord Prolapse Guideline v3. 01May 19.doc Policy categories for Trust’s internal Tags for Trust’s internal website (Bob) website (Bob) Cord, accidents, prolapse Maternity Services Maternity Page 1 of 11 Umbilical Cord Prolapse Guideline CONTENTS Document Control .................................................................................................... 1 1. Introduction -

Managing Complications in Pregnancy and Childbirth Fetal
IMPAC FEtal distress in labour WHO Home | Reproductive Health Home | HRP | What's new | Resources | Contact | Search Department of Reproductive Health and Research (RHR), World Health Organization z Abbreviations Managing Complications in Pregnancy and Childbirth z Index A guide for midwives and doctors z List of diagnoses z MCPC Home Section 2 - Symptoms Clinical principles Rapid initial assessment Fetal distress in labour Talking with women and their families Emotional and psychological support PROBLEMS Emergencies z Abnormal fetal heart rate (less than 100 or more than 180 beats per minute). General care principles Clinical use of blood, blood products and z Thick meconium-stained amniotic fluid. replacement fluids Antibiotic therapy Anaesthesia and analgesia GENERAL MANAGEMENT Operative care principles z Prop up the woman or place her on her left side. Normal Labour and childbirth Newborn care principles z Stop oxytocin if it is being administered. Provider and community linkages ABNORMAL FETAL HEART RATE Symptoms BOX S-7 Abnormal fetal heart rate Shock Vaginal bleeding in early pregnancy z A normal fetal heart rate may slow during a contraction but usually recovers to normal as Vaginal bleeding in later pregnancy and soon as the uterus relaxes. labour z A very slow fetal heart rate in the absence of contractions or persisting after contractions is Vaginal bleeding after childbirth suggestive of fetal distress. Headache, blurred vision, convulsions or loss of consciousness, elevated blood z A rapid fetal heart rate may be a response to maternal fever, drugs causing rapid maternal pressure heart rate (e.g. tocolytic drugs), hypertension or amnionitis. In the absence of a rapid maternal Unsatisfactory progress of Labour heart rate, a rapid fetal heart rate should be considered a sign of fetal distress. -

Management of Prolonged Decelerations ▲
OBG_1106_Dildy.finalREV 10/24/06 10:05 AM Page 30 OBGMANAGEMENT Gary A. Dildy III, MD OBSTETRIC EMERGENCIES Clinical Professor, Department of Obstetrics and Gynecology, Management of Louisiana State University Health Sciences Center New Orleans prolonged decelerations Director of Site Analysis HCA Perinatal Quality Assurance Some are benign, some are pathologic but reversible, Nashville, Tenn and others are the most feared complications in obstetrics Staff Perinatologist Maternal-Fetal Medicine St. Mark’s Hospital prolonged deceleration may signal ed prolonged decelerations is based on bed- Salt Lake City, Utah danger—or reflect a perfectly nor- side clinical judgment, which inevitably will A mal fetal response to maternal sometimes be imperfect given the unpre- pelvic examination.® BecauseDowden of the Healthwide dictability Media of these decelerations.” range of possibilities, this fetal heart rate pattern justifies close attention. For exam- “Fetal bradycardia” and “prolonged ple,Copyright repetitive Forprolonged personal decelerations use may onlydeceleration” are distinct entities indicate cord compression from oligohy- In general parlance, we often use the terms dramnios. Even more troubling, a pro- “fetal bradycardia” and “prolonged decel- longed deceleration may occur for the first eration” loosely. In practice, we must dif- IN THIS ARTICLE time during the evolution of a profound ferentiate these entities because underlying catastrophe, such as amniotic fluid pathophysiologic mechanisms and clinical 3 FHR patterns: embolism or uterine rupture during vagi- management may differ substantially. What would nal birth after cesarean delivery (VBAC). The problem: Since the introduction In some circumstances, a prolonged decel- of electronic fetal monitoring (EFM) in you do? eration may be the terminus of a progres- the 1960s, numerous descriptions of FHR ❙ Complete heart sion of nonreassuring fetal heart rate patterns have been published, each slight- block (FHR) changes, and becomes the immedi- ly different from the others. -

Role of Internal Podalic Version in Developing Countries a Mahendru, O Ogueh, K Gajjar, C Rawat
The Internet Journal of Gynecology and Obstetrics ISPUB.COM Volume 6 Number 1 Role Of Internal Podalic Version In Developing Countries A Mahendru, O Ogueh, K Gajjar, C Rawat Citation A Mahendru, O Ogueh, K Gajjar, C Rawat. Role Of Internal Podalic Version In Developing Countries. The Internet Journal of Gynecology and Obstetrics. 2005 Volume 6 Number 1. Abstract Objective: To study the role of internal podalic version in the management of undiagnosed transverse lie in labour in developing countries and its place in the management of 2nd twin. Materials and methods: This is a retrospective case series study of 41 cases of internal podalic version from January 1997 to August 2002. The data was collected from the labour ward register and was analysed. Main outcome measures: The primary outcome was analysis of the indications of internal podalic version in developing countries. The secondary outcome was to assess the success and outcome of version and the factors affecting the success of this almost lost art by analysing these cases. Results: There were 41 (15.8%) cases of internal podalic version out of 261 cases of transverse lie. All these cases were undiagnosed transverse lie with intrauterine fetal death in labour. None of these cases had any form of antenatal care. Half of the cases were more than 37 weeks gestation. 31 (76%) cases were with >7cm cervical dilatation. Version resulted into minor complications like 5 cases of cervical tears, 7 cases of para- urethral and vaginal tears. No case of rupture uterus or obstetric shock was reported. There were 2 cases of failure of version one of which was followed by LSCS and the other by vaginal birth following reductive surgery. -

A Guide to Obstetrical Coding Production of This Document Is Made Possible by Financial Contributions from Health Canada and Provincial and Territorial Governments
ICD-10-CA | CCI A Guide to Obstetrical Coding Production of this document is made possible by financial contributions from Health Canada and provincial and territorial governments. The views expressed herein do not necessarily represent the views of Health Canada or any provincial or territorial government. Unless otherwise indicated, this product uses data provided by Canada’s provinces and territories. All rights reserved. The contents of this publication may be reproduced unaltered, in whole or in part and by any means, solely for non-commercial purposes, provided that the Canadian Institute for Health Information is properly and fully acknowledged as the copyright owner. Any reproduction or use of this publication or its contents for any commercial purpose requires the prior written authorization of the Canadian Institute for Health Information. Reproduction or use that suggests endorsement by, or affiliation with, the Canadian Institute for Health Information is prohibited. For permission or information, please contact CIHI: Canadian Institute for Health Information 495 Richmond Road, Suite 600 Ottawa, Ontario K2A 4H6 Phone: 613-241-7860 Fax: 613-241-8120 www.cihi.ca [email protected] © 2018 Canadian Institute for Health Information Cette publication est aussi disponible en français sous le titre Guide de codification des données en obstétrique. Table of contents About CIHI ................................................................................................................................. 6 Chapter 1: Introduction .............................................................................................................. -

Gtg-No-20B-Breech-Presentation.Pdf
Guideline No. 20b December 2006 THE MANAGEMENT OF BREECH PRESENTATION This is the third edition of the guideline originally published in 1999 and revised in 2001 under the same title. 1. Purpose and scope The aim of this guideline is to provide up-to-date information on methods of delivery for women with breech presentation. The scope is confined to decision making regarding the route of delivery and choice of various techniques used during delivery. It does not include antenatal or postnatal care. External cephalic version is the topic of a separate RCOG Green-top Guideline No. 20a: ECV and Reducing the Incidence of Breech Presentation. 2. Background The incidence of breech presentation decreases from about 20% at 28 weeks of gestation to 3–4% at term, as most babies turn spontaneously to the cephalic presentation. This appears to be an active process whereby a normally formed and active baby adopts the position of ‘best fit’ in a normal intrauterine space. Persistent breech presentation may be associated with abnormalities of the baby, the amniotic fluid volume, the placental localisation or the uterus. It may be due to an otherwise insignificant factor such as cornual placental position or it may apparently be due to chance. There is higher perinatal mortality and morbidity with breech than cephalic presentation, due principally to prematurity, congenital malformations and birth asphyxia or trauma.1,2 Caesarean section for breech presentation has been suggested as a way of reducing the associated perinatal problems2,3 and in many countries in Northern Europe and North America caesarean section has become the normal mode of breech delivery. -

Skin Eruptions Specific to Pregnancy: an Overview
DOI: 10.1111/tog.12051 Review The Obstetrician & Gynaecologist http://onlinetog.org Skin eruptions specific to pregnancy: an overview a, b Ajaya Maharajan MBBS DGO MRCOG, * Christina Aye BMBCh MA Hons MRCOG, c d Ravi Ratnavel DM(Oxon) FRCP(UK), Ekaterina Burova FRCP CMSc (equ. PhD) aConsultant in Obstetrics and Gynaecology, Luton and Dunstable University Hospital, Lewsey Road, Luton, Bedfordshire LU4 0DZ, UK bST5 in Obstetrics and Gynaecology, John Radcliffe Hospital, Headley Way, Headington, Oxford OX3 9DU, UK cConsultant Dermatologist, Buckinghamshire Health Care, Mandeville Road, Aylesbury, Buckinghamshire HP21 8AL, UK dConsultant Dermatologist, Skin Cancer Lead for Bedford Hospital, Bedford Hospital NHS Trust, South Wing, Kempston Road, Bedford MK42 9DJ, UK *Correspondence: Ajaya Maharajan. Email: [email protected] Accepted on 31 January 2013 Key content Learning objectives Pregnancy results in various physiological skin changes. To understand the physiological skin changes in pregnancy. As a consequence, some common dermatoses can present more To identify the skin conditions that require appropriate referral. frequently in pregnant women. In addition, there are a number To be able to take a history, to diagnose the skin eruptions unique to of skin eruptions unique to pregnancy. pregnancy, undertake appropriate investigations and first-line The aetiology of physiological skin changes in pregnancy is management, and understand the criteria for referral to a uncertain but is thought to be due to hormonal and physical dermatologist. changes of pregnancy. Keywords: atopic eruption of pregnancy / intrahepatic cholestasis The four dermatoses of pregnancy are: atopic eruption of pregnancy / pemphigoid gestastionis / polymorphic eruption of of pregnancy, pemphigoid gestationis, polymorphic pregnancy / skin eruptions eruption of pregnancy and intrahepatic cholestasis of pregnancy. -

Internal Podalic Version and Extraction : the History, Development, and Modern Day Application
University of Nebraska Medical Center DigitalCommons@UNMC MD Theses Special Collections 5-1-1964 Internal podalic version and extraction : the history, development, and modern day application Thomas C. Bush University of Nebraska Medical Center This manuscript is historical in nature and may not reflect current medical research and practice. Search PubMed for current research. Follow this and additional works at: https://digitalcommons.unmc.edu/mdtheses Part of the Medical Education Commons Recommended Citation Bush, Thomas C., "Internal podalic version and extraction : the history, development, and modern day application" (1964). MD Theses. 6. https://digitalcommons.unmc.edu/mdtheses/6 This Thesis is brought to you for free and open access by the Special Collections at DigitalCommons@UNMC. It has been accepted for inclusion in MD Theses by an authorized administrator of DigitalCommons@UNMC. For more information, please contact [email protected]. INTEIU~AL PODALIC VERSION ~1) EXTRACTION The History, Development and Modern Day Application Thomas Charles Bush Submitted in Partial Fulfillment for the Degree of Doctor of Medicine College of Medicine, University of Nebraska January 23, 1964 Omaha, Nebraska Index Part I A. Introduction and definition---page 1 B. History and development of internal podalic version-- pp. 1-3 1) Early practice by Pare and Soranus---pp. 1-2 2) Early publications---page 2 3) Braxton-Hicks method----page 2 4) llO .. case study of' Braxton-Hicks method---page 3 C. Potter's method and contributions pp. 3-6 1) Potter's method---pp. 3-5 2) Potter's results---page 5 a) shortening of labor---page 5 bJ complica~ions and ind~cations page 0 D.