A Review of Introduced Cervids in Chile
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

O18934 Plard, F., Bonenfant, C. and Gaillard, JM 2011
Oikos O18934 Plard, F., Bonenfant, C. and Gaillard, J. M. 2011. Re- visiting the allometry of antlers among deer species: male-male sexual competition as a driver. – Oikos 120: 601–606. Table A1. Data basis. Sexual size dimorphism (SSD) was measured by the residuals of the regression of male body mass against female body mass. Breeding group sizes (BGS) were divided into three categories: group of one or two animals (A), group of three, four and five animals (B), and groups larger than five animals (C). The different mating tactics (MT) were harem (H), territorial (T) and tending (F). Non-available mating tactics (NA) and monogamous species (M) are indicated. Sub-family Genus Species Common name Male Female SSD BGS MT Ref Antler Body Body length mass mass Cervinae Axis axis chital 845 89.5 39 0.58 C F 1,2,3 Cervinae Axis porcinus hog deer 399 41 31 0.07 C F 2,3,4,5 Cervinae Cervus albirostris white-lipped deer 1150 204 125 0.06 C H 1,6 Cervinae Cervus canadensis wapiti 1337 350 250 –0.21 C H 3,8,9 Cervinae Cervus duvaucelii barasingha 813 236 145 0.03 C H 1,3,10,11 Cervinae Cervus elaphus red deer 936 250 125 0.34 C H 1,2,3 Cervinae Cervus eldi Eld’s deer 971.5 105 67 0.12 C H 1,2,3 Cervinae Cervus nippon sika deer 480 52 37 0.1 B T 1,2,3,9,12,13 Cervinae Cervus timorensis Timor deer 675 95.5 53 0.29 C H 1,9 Cervinae Cervus unicolor sambar 1049 192 146 –0.18 B T 1,2,3,7 Cervinae Dama dama fallow deer 615 67 44 0.15 C T 1,2,3,14 Cervinae Elaphurus davidianus Père David’s deer 737 214 159 –0.17 C H 1,2,3 Muntiacinae Elaphodus cephalophus -

An Analysis of the Phylogenetic Relationship of Thai Cervids Inferred from Nucleotide Sequences of Protein Kinase C Iota (PRKCI) Intron
Kasetsart J. (Nat. Sci.) 43 : 709 - 719 (2009) An Analysis of the Phylogenetic Relationship of Thai Cervids Inferred from Nucleotide Sequences of Protein Kinase C Iota (PRKCI) Intron Kanita Ouithavon1, Naris Bhumpakphan2, Jessada Denduangboripant3, Boripat Siriaroonrat4 and Savitr Trakulnaleamsai5* ABSTRACT The phylogenetic relationship of five Thai cervid species (n=21) and four spotted deer (Axis axis, n=4), was determined based on nucleotide sequences of the intron region of the protein kinase C iota (PRKCI) gene. Blood samples were collected from seven captive breeding centers in Thailand from which whole genomic DNA was extracted. Intron1 sequences of the PRKCI nuclear gene were amplified by a polymerase chain reaction, using L748 and U26 primers. Approximately 552 base pairs of all amplified fragments were analyzed using the neighbor-joining distance matrix method, and 19 parsimony- informative sites were analyzed using the maximum parsimony approach. Phylogenetic analyses using the subfamily Muntiacinae as outgroups for tree rooting indicated similar topologies for both phylogenetic trees, clearly showing a separation of three distinct genera of Thai cervids: Muntiacus, Cervus, and Axis. The study also found that a phylogenetic relationship within the genus Axis would be monophyletic if both spotted deer and hog deer were included. Hog deer have been conventionally classified in the genus Cervus (Cervus porcinus), but this finding supports a recommendation to reclassify hog deer in the genus Axis. Key words: Thailand, the family Cervidae, protein kinase C iota (PRKCI) intron, phylogenetic tree, taxonomy INTRODUCTION 1987; Gentry, 1994). Cervids, or what is commonly called “deer,” are mostly characterized The family Cervidae is one of the most by antlers with a bony inner core and velvet skin specious families of artiodactyls, with an extensive cover. -

Bushmeat Hunting and Extinction Risk to the World’S Rsos.Royalsocietypublishing.Org Mammals
Downloaded from http://rsos.royalsocietypublishing.org/ on October 26, 2017 Bushmeat hunting and extinction risk to the world’s rsos.royalsocietypublishing.org mammals 1,2 4,5 Research William J. Ripple , Katharine Abernethy , Matthew G. Betts1,2, Guillaume Chapron6, Cite this article: Ripple WJ et al.2016 Bushmeat hunting and extinction risk to the Rodolfo Dirzo7, Mauro Galetti8,9, Taal Levi1,2,3, world’s mammals R. Soc. open sci. 3: 160498. 10,11 12 http://dx.doi.org/10.1098/rsos.160498 Peter A. Lindsey , David W. Macdonald , Brian Machovina13, Thomas M. Newsome1,14,15,16, Carlos A. Peres17, Arian D. Wallach18, Received: 10 July 2016 Accepted: 20 September 2016 Christopher Wolf1,2 and Hillary Young19 1GlobalTrophic Cascades Program, Department of Forest Ecosystems and Society, 2Forest Biodiversity Research Network, Department of Forest Ecosystems and Society, and 3Department of Fisheries and Wildlife, Oregon State University, Corvallis, Subject Category: OR 97331, USA Biology (whole organism) 4School of Natural Sciences, University of Stirling, Stirling FK9 4LA, UK 5Institut de Recherche en Ecologie Tropicale, CENAREST, BP 842 Libreville, Gabon Subject Areas: 6Grimsö Wildlife Research Station, Department of Ecology, Swedish University of ecology Agricultural Sciences, 73091 Riddarhyttan, Sweden 7Department of Biology, Stanford University, Stanford, CA 94305, USA Keywords: 8Universidade Estadual Paulista (UNESP), Instituto Biociências, Departamento de wild meat, bushmeat, hunting, mammals, Ecologia, 13506-900 Rio Claro, São Paulo, Brazil extinction 9Department of Bioscience, Ecoinformatics and Biodiversity, Aarhus University, 8000 Aarhus, Denmark 10Panthera, 8 West 40th Street, 18th Floor, New York, NY 10018, USA 11 Author for correspondence: Mammal Research Institute, Department of Zoology and Entomology, University of William J. -

Pudu in a Chilean National Park
547 Pudu in a Chilean National Park Gary 8. Wetterberg The Chilean pudu Pudu pudu, the smallest American deer, is on the world list of endangered species in the IUCN Red Data Book. One of its few remaining refuges is in the Vicente Perez Rosales National Park. This is in the Lake District of southern Chile, the 'Switzerland of South America', between the Puyehue National Park to the north, and the Nahuel Huapi National Park in Argentina on the east. There are very few records on the fauna of this park, which covers 243,000 hectares, and is part of the Patagonian Subdivision of the Neotropical Faunal Region. Like an Island In many ways, Chile is like an island, cut off by the Atacama Desert on the north, the Andes to the east, the Patagonian ice fields and fiords to the south, and the Pacific on the west. This geo- graphical isolation has permitted the development of a unique biota, and Chilean wildlife exhibits some of the characteristics of island fauna such as narrow endemics and few competitors. The pudu is descended from the deer that migrated from North America in the late Tertiary period (Simpson 1950). The species is primarily of Chilean origin and distribution, although it is frequently encountered in adjacent areas of Argentina, and is present in Bolivia (Walker, 1964). It was discovered and named in 1782 by the Jesuit Juan Ignacio Molina, the 'father of Chilean natural history' (Osgood, 1943). Other species of the genus are found in Ecuador and Peru (Grimwood, 1968), and Brazil (Hershkovitz, 1958). -

Capture, Restraint and Transport Stress in Southern Chamois (Rupicapra Pyrenaica)
Capture,Capture, restraintrestraint andand transporttransport stressstress ininin SouthernSouthern chamoischamois ((RupicapraRupicapra pyrenaicapyrenaica)) ModulationModulation withwith acepromazineacepromazine andand evaluationevaluation usingusingusing physiologicalphysiologicalphysiological parametersparametersparameters JorgeJorgeJorge RamónRamónRamón LópezLópezLópez OlveraOlveraOlvera 200420042004 Capture, restraint and transport stress in Southern chamois (Rupicapra pyrenaica) Modulation with acepromazine and evaluation using physiological parameters Jorge Ramón López Olvera Bellaterra 2004 Esta tesis doctoral fue realizada gracias a la financiación de la Comisión Interministerial de Ciencia y Tecnología (proyecto CICYT AGF99- 0763-C02) y a una beca predoctoral de Formación de Investigadores de la Universidad Autónoma de Barcelona, y contó con el apoyo del Departament de Medi Ambient de la Generalitat de Catalunya. Los Doctores SANTIAGO LAVÍN GONZÁLEZ e IGNASI MARCO SÁNCHEZ, Catedrático de Universidad y Profesor Titular del Área de Conocimiento de Medicina y Cirugía Animal de la Facultad de Veterinaria de la Universidad Autónoma de Barcelona, respectivamente, CERTIFICAN: Que la memoria titulada ‘Capture, restraint and transport stress in Southern chamois (Rupicapra pyrenaica). Modulation with acepromazine and evaluation using physiological parameters’, presentada por el licenciado Don JORGE R. LÓPEZ OLVERA para la obtención del grado de Doctor en Veterinaria, se ha realizado bajo nuestra dirección y, considerándola satisfactoriamente -

Arabian Tahr in Oman Paul Munton
Arabian Tahr in Oman Paul Munton Arabian tahr are confined to Oman, with a population of under 2000. Unlike other tahr species, which depend on grass, Arabian tahr require also fruits, seeds and young shoots. The areas where these can be found in this arid country are on certain north-facing mountain slopes with a higher rainfall, and it is there that reserves to protect this tahr must be made. The author spent two years in Oman studying the tahr. The Arabian tahr Hemitragus jayakari today survives only in the mountains of northern Oman. A goat-like animal, it is one of only three surviving species of a once widespread genus; the other two are the Himalayan and Nilgiri tahrs, H. jemlahicus and H. hylocrius. In recent years the government of the Sultanate of Oman has shown great interest in the country's wildlife, and much conservation work has been done. From April 1976 to April 1978 I was engaged jointly by the Government, WWF and IUCN on a field study of the tahr's ecology, and in January 1979 made recommendations for its conservation, which were presented to the Government. Arabian tahr differ from the other tahrs in that they feed selectively on fruits, seeds and young shoots as well as grass. Their optimum habitat is found on the north-facing slopes of the higher mountain ranges of northern Oman, where they use all altitudes between sea level and 2000 metres. But they prefer the zone between 1000 and 1800m where the vegetation is especially diverse, due to the special climate of these north-facing slopes, with their higher rainfall, cooler temperatures, and greater shelter from the sun than in the drought conditions that are otherwise typical of this arid zone. -

Anaplasma Phagocytophilum and Babesia Species Of
pathogens Article Anaplasma phagocytophilum and Babesia Species of Sympatric Roe Deer (Capreolus capreolus), Fallow Deer (Dama dama), Sika Deer (Cervus nippon) and Red Deer (Cervus elaphus) in Germany Cornelia Silaghi 1,2,*, Julia Fröhlich 1, Hubert Reindl 3, Dietmar Hamel 4 and Steffen Rehbein 4 1 Institute of Comparative Tropical Medicine and Parasitology, Ludwig-Maximilians-Universität München, Leopoldstr. 5, 80802 Munich, Germany; [email protected] 2 Institute of Infectology, Friedrich-Loeffler-Institut, Südufer 10, 17493 Greifswald Insel Riems, Germany 3 Tierärztliche Fachpraxis für Kleintiere, Schießtrath 12, 92709 Moosbach, Germany; [email protected] 4 Boehringer Ingelheim Vetmedica GmbH, Kathrinenhof Research Center, Walchenseestr. 8-12, 83101 Rohrdorf, Germany; [email protected] (D.H.); steff[email protected] (S.R.) * Correspondence: cornelia.silaghi@fli.de; Tel.: +49-0-383-5171-172 Received: 15 October 2020; Accepted: 18 November 2020; Published: 20 November 2020 Abstract: (1) Background: Wild cervids play an important role in transmission cycles of tick-borne pathogens; however, investigations of tick-borne pathogens in sika deer in Germany are lacking. (2) Methods: Spleen tissue of 74 sympatric wild cervids (30 roe deer, 7 fallow deer, 22 sika deer, 15 red deer) and of 27 red deer from a farm from southeastern Germany were analyzed by molecular methods for the presence of Anaplasma phagocytophilum and Babesia species. (3) Results: Anaplasma phagocytophilum and Babesia DNA was demonstrated in 90.5% and 47.3% of the 74 combined wild cervids and 14.8% and 18.5% of the farmed deer, respectively. Twelve 16S rRNA variants of A. phagocytophilum were delineated. -

Ecology of Red Deer a Research Review Relevant to Their Management in Scotland
Ecologyof RedDeer A researchreview relevant to theirmanagement in Scotland Instituteof TerrestrialEcology Natural EnvironmentResearch Council á á á á á Natural Environment Research Council Institute of Terrestrial Ecology Ecology of Red Deer A research review relevant to their management in Scotland Brian Mitchell, Brian W. Staines and David Welch Institute of Terrestrial Ecology Banchory iv Printed in England by Graphic Art (Cambridge) Ltd. ©Copyright 1977 Published in 1977 by Institute of Terrestrial Ecology 68 Hills Road Cambridge CB2 11LA ISBN 0 904282 090 Authors' address: Institute of Terrestrial Ecology Hill of Brathens Glassel, Banchory Kincardineshire AB3 4BY Telephone 033 02 3434. The Institute of Terrestrial Ecology (ITE) was established in 1973, from the former Nature Conservancy's research stations and staff, joined later by the Institute of Tree Biology and the Culture Centre of Algae and Protozoa. ITE contributes to and draws upon the collective knowledge of the fourteen sister institutes which make up the Natural Environment Research Council, spanning all the environmental sciences. The Institute studies the factors determining the structure, composition and processes of land and freshwater systems, and of individual plant and animal species. It is developing a Sounder scientific basis for predicting and modelling environmental trends arising from natural or man-made change. The results of this research are available to those responsible for the protection, management and wise use of our natural resources. Nearly half of ITE'Swork is research commissioned by customers, such as the Nature Conservancy Council who require information for wildlife conservation, the Forestry Commission and the Department of the Environment. The remainder is fundamental research supported by NERC. -
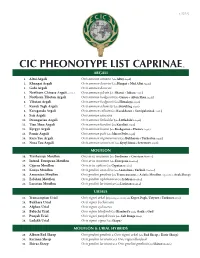
Cic Pheonotype List Caprinae©
v. 5.25.12 CIC PHEONOTYPE LIST CAPRINAE © ARGALI 1. Altai Argali Ovis ammon ammon (aka Altay Argali) 2. Khangai Argali Ovis ammon darwini (aka Hangai & Mid Altai Argali) 3. Gobi Argali Ovis ammon darwini 4. Northern Chinese Argali - extinct Ovis ammon jubata (aka Shansi & Jubata Argali) 5. Northern Tibetan Argali Ovis ammon hodgsonii (aka Gansu & Altun Shan Argali) 6. Tibetan Argali Ovis ammon hodgsonii (aka Himalaya Argali) 7. Kuruk Tagh Argali Ovis ammon adametzi (aka Kuruktag Argali) 8. Karaganda Argali Ovis ammon collium (aka Kazakhstan & Semipalatinsk Argali) 9. Sair Argali Ovis ammon sairensis 10. Dzungarian Argali Ovis ammon littledalei (aka Littledale’s Argali) 11. Tian Shan Argali Ovis ammon karelini (aka Karelini Argali) 12. Kyrgyz Argali Ovis ammon humei (aka Kashgarian & Hume’s Argali) 13. Pamir Argali Ovis ammon polii (aka Marco Polo Argali) 14. Kara Tau Argali Ovis ammon nigrimontana (aka Bukharan & Turkestan Argali) 15. Nura Tau Argali Ovis ammon severtzovi (aka Kyzyl Kum & Severtzov Argali) MOUFLON 16. Tyrrhenian Mouflon Ovis aries musimon (aka Sardinian & Corsican Mouflon) 17. Introd. European Mouflon Ovis aries musimon (aka European Mouflon) 18. Cyprus Mouflon Ovis aries ophion (aka Cyprian Mouflon) 19. Konya Mouflon Ovis gmelini anatolica (aka Anatolian & Turkish Mouflon) 20. Armenian Mouflon Ovis gmelini gmelinii (aka Transcaucasus or Asiatic Mouflon, regionally as Arak Sheep) 21. Esfahan Mouflon Ovis gmelini isphahanica (aka Isfahan Mouflon) 22. Larestan Mouflon Ovis gmelini laristanica (aka Laristan Mouflon) URIALS 23. Transcaspian Urial Ovis vignei arkal (Depending on locality aka Kopet Dagh, Ustyurt & Turkmen Urial) 24. Bukhara Urial Ovis vignei bocharensis 25. Afghan Urial Ovis vignei cycloceros 26. -

Dama Dama. by George A
MAMMALIAN SPECIES No. 317, pp. 1-8,3 figs. Dama dama. By George A. Feldharner, Kelly C. Farris-Renner, and Celeste M. Barker Published 27 December 1988 by The American Society of Mammalogists Dama dama (Linnaeus, 1758) Detailed descripti ons of the skull and dentition of European fallow deer are in Flerov (19 52), and Harrison (1968) described Persian Fallow Deer fallow deer. Cercus dum a Linnaeus, 1758 :67. T ype locality Sweden (introduced). Downloaded from https://academic.oup.com/mspecies/article/doi/10.2307/3504141/2600626 by guest on 29 September 2021 Ty pe spec ies of Duma Frisch. 1775 validated by plenary GENERAL CHARACTERS. Pelage coloration is the most powers. var iable of any spec ies of deer, with four main color varieties: white. Plat vccros plinii Zimmer ma n, 1780: 129. Renamin g of duma. men il, common (typical), and black (Chapm an and Chapm an. 1975). Cercus platvccros G. Cuvier , 1798:160. Renaming of dama. Int ermediat e pelage colors are cream. sandy , silver-grey, and sooty C NI'IIS mauricus F. Cuvier, 18 16:72. No localit y given. (Whitehead. 1972). Typical pelage is darker on the dorsal surface Ccr ru s (I)ama) m esopot amicus Brooke. 1875:26 4. Type localit y than the ventral sur face . ches t. and lower legs. A black dor sal stripe Khuzistan, Luristan (Persia). Ir an . ext end s from the na pe of the neck to the tip of the tail and around the upper edge of the white rump patch. T ypically. white spots are CONTEXT AND CONTENT. -

Species Fact Sheet: Sika Deer (Cervus Nippon) [email protected] 023 8023 7874
Species Fact Sheet: Sika Deer (Cervus nippon) [email protected] www.mammal.org.uk 023 8023 7874 Quick Facts Recognition: A medium-sized deer. Has a similar spotted coat to fallow deer in summer, but usually is rougher, thicker, dark grey-brown in winter. Tail is shorter than fallow deer, but with similar white “target” and black margins. Usually has a distinctive “furrowed brow” look, and if seen well, evident white spots on the limbs, marking the site of pedal glands. Males have rounded, not pamate, antlers, looking like a small version of a red deer stag’s antlers. Size: 138-179 cm; Tail length: 14-21cm; Shoulder height 50-120 cm. Weight: Males 40-63kg; females 31-44kg. Life Span: Maximum recorded lifespan in captivity is 26 years; 16 in the wild. Distribution & Habitat Sika are native to SE China, including Taiwan, Korea and Japan. It was introduced to Powerscourt Park, Co Wicklow, Ireland, in 1860, and to London Zoo. Sika then spread to many other parks and escaped or were deliberately released; in some cases they were deliberately released into surrounding woodlands to be hunted on horseback. This resulted in feral populations S England (especially Dorset and the New Forest), in the Forest of Bowland and S Cumbria, and, especially, in Scotland. It is still spreading. Its preference for conifer plantations, especially the thick young stages, has been a big advantage to it. It can reach densities up to 45/km2 in prime habitat. General Ecology Behaviour They typically live in small herds of 6-7 animals, at least in more open habitats, but in dense cover may only live in small groups of 1-3 only. -

The European Fallow Deer (Dama Dama Dama)
Heredity (2017) 119, 16–26 OPEN Official journal of the Genetics Society www.nature.com/hdy ORIGINAL ARTICLE Strong population structure in a species manipulated by humans since the Neolithic: the European fallow deer (Dama dama dama) KH Baker1, HWI Gray1, V Ramovs1, D Mertzanidou2,ÇAkın Pekşen3,4, CC Bilgin3, N Sykes5 and AR Hoelzel1 Species that have been translocated and otherwise manipulated by humans may show patterns of population structure that reflect those interactions. At the same time, natural processes shape populations, including behavioural characteristics like dispersal potential and breeding system. In Europe, a key factor is the geography and history of climate change through the Pleistocene. During glacial maxima throughout that period, species in Europe with temperate distributions were forced south, becoming distributed among the isolated peninsulas represented by Anatolia, Italy and Iberia. Understanding modern patterns of diversity depends on understanding these historical population dynamics. Traditionally, European fallow deer (Dama dama dama) are thought to have been restricted to refugia in Anatolia and possibly Sicily and the Balkans. However, the distribution of this species was also greatly influenced by human-mediated translocations. We focus on fallow deer to better understand the relative influence of these natural and anthropogenic processes. We compared modern fallow deer putative populations across a broad geographic range using microsatellite and mitochondrial DNA loci. The results revealed highly insular populations, depauperate of genetic variation and significantly differentiated from each other. This is consistent with the expectations of drift acting on populations founded by small numbers of individuals, and reflects known founder populations in the north.