Guideline for Complete Blood Count in Medical Laboratories: Effects of Preanalytical Parameters
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

VIROLOGY for MEDICAL LABORATORY STUDENTS Mwamisi, Muthwii, Mwala, Mokua
VIROLOGY for MEDICAL LABORATORY STUDENTS Mwamisi, Muthwii, Mwala, Mokua To the Reader Virology is an important subject in Medical Laboratory Sciences. Medical Laboratory Medicine puts a technologist/technician in the fore front in diagnosis and proper interpretation of the correct diseases by a clinician. Research on viruses, therefore, has gained a lot of momentum in recent years due to the Medical importance they contribute to the field of medicine. A lot of this work goes through the medical laboratory Department where it is handled by the medical laboratory students, especially, on practical experience. The purpose of this manual is, therefore, to provide to the student adequate up-to-date information in preparation for the work that the student will perform in any Medical Laboratory Department, whether research or routine work. We hope this information will not only be useful to the medical laboratory Students or staff, but!also will be a reference material for others in Health or health related field. Joseph M. Mwamisi, PhD Samson M. Muthwii PhD Denis M. Mwala M.Se. John M. Mokua M.Se., ) 2 TABLE OF CONTENTS 1. VIROLOGY 6 1.1 Definition of Virology 6 1.2 Definition of Viruses 6 1.3 Differences between Bacteria and Viruses 7 1.4 Morphology of Viruses 7 2. CLASSIFICATION OF VIRUSES 9 2.1 Classification 9 Epidemiological Analysis 9 Physical and Chemical Properties Analysis 9 DNA Viruses 9 RNA Viruses 9 Enteric Viruses 10 Respiratory Viruses ' 10 Arthropod-Borne Viruses (Arboviruses) 10 2.2. DNA Viruses 10 Poxvirusae 10 Ridoviridae 10 2.3. RNA Viruses 10 Picornaviridae 11 3. -

Human Physiology an Integrated Approach
Gas Exchange and Transport Gas Exchange in the Lungs and Tissues 18 Lower Alveolar P Decreases Oxygen Uptake O2 Diff usion Problems Cause Hypoxia Gas Solubility Aff ects Diff usion Gas Transport in the Blood Hemoglobin Binds to Oxygen Oxygen Binding Obeys the Law of Mass Action Hemoglobin Transports Most Oxygen to the Tissues P Determines Oxygen-Hb Binding O2 Oxygen Binding Is Expressed As a Percentage Several Factors Aff ect Oxygen-Hb Binding Carbon Dioxide Is Transported in Three Ways Regulation of Ventilation Neurons in the Medulla Control Breathing Carbon Dioxide, Oxygen, and pH Infl uence Ventilation Protective Refl exes Guard the Lungs Higher Brain Centers Aff ect Patterns of Ventilation The successful ascent of Everest without supplementary oxygen is one of the great sagas of the 20th century. — John B. West, Climbing with O’s , NOVA Online (www.pbs.org) Background Basics Exchange epithelia pH and buff ers Law of mass action Cerebrospinal fl uid Simple diff usion Autonomic and somatic motor neurons Structure of the brain stem Red blood cells and Giant liposomes hemoglobin of pulmonary Blood-brain barrier surfactant (40X) From Chapter 18 of Human Physiology: An Integrated Approach, Sixth Edition. Dee Unglaub Silverthorn. Copyright © 2013 by Pearson Education, Inc. All rights reserved. 633 Gas Exchange and Transport he book Into Thin Air by Jon Krakauer chronicles an ill- RUNNING PROBLEM fated trek to the top of Mt. Everest. To reach the summit of Mt. Everest, climbers must pass through the “death zone” T High Altitude located at about 8000 meters (over 26,000 ft ). Of the thousands of people who have attempted the summit, only about 2000 have been In 1981 a group of 20 physiologists, physicians, and successful, and more than 185 have died. -

Medical Laboratory Scientist LIMITED ENTRY BACHELOR of APPLIED SCIENCE (BAS) REQUIRED CREDITS: 120 DEGREE CODE: MLS-BAS
MEDICAL LABORATORY PROGRAM Medical Laboratory Scientist LIMITED ENTRY BACHELOR OF APPLIED SCIENCE (BAS) REQUIRED CREDITS: 120 DEGREE CODE: MLS-BAS This is a limited-entry program. Some of these courses are program prerequisites and MUST be completed before a student is considered eligible for entry into the Program. Students MUST attend a Health Programs orientation and meet with a Health Programs advisor for additional advisement. DESCRIPTION The Medical Laboratory Scientist (MLS) is an important member of the health care team in hospitals, clinics, medical research and teaching centers, and is an indispensable participant with physicians in providing critical diagnostic information. The MLS functions as a dependable, ambitious and highly motivated professional capable of handling high stress situations with ease and confidence. The Medical Laboratory Scientist performs and interprets diagnostic laboratory procedures using state-of-the-art instrumentation to aid in the detection, diagnosis, and treatment of disease; monitors the standards of accuracy and precision in the performance of tests; performs routine maintenance; analyzes and corrects instrument problems; researches, evaluates, and implements new procedures; and may be responsible for fiscal/personnel management of laboratory. The Bachelor of Applied Science degree in Medical Laboratory Scientist combines academic and laboratory courses on campus with practical experience at clinical affiliate sites. The program is accredited by the National Accrediting Agency for Clinical Laboratory Sciences (NAACLS), 5600 N. River Road, Suite 720, Rosemont, IL 60018-5119, (877) 939-3597. Students successfully completing the program are eligible to take a national certifying examination. STUDENT LEARNING OUTCOMES • Select appropriate courses of action in accordance with established laboratory procedures. -

Cell Counting and Viability Assessment of 2D and 3D Cell
Piccinini et al. Biological Procedures Online (2017) 19:8 DOI 10.1186/s12575-017-0056-3 METHODOLOGY Open Access Cell Counting and Viability Assessment of 2D and 3D Cell Cultures: Expected Reliability of the Trypan Blue Assay Filippo Piccinini1*† , Anna Tesei1†, Chiara Arienti1 and Alessandro Bevilacqua2,3 Abstract Background: Whatever the target of an experiment in cell biology, cell counting and viability assessment are always computed. The Trypan Blue (TB) assay was proposed about a century ago and is still the most widely used method to perform cell viability analysis. Furthermore, the combined use of TB with a haemocytometer is also considered the standard approach to estimate cell population density. There are numerous research articles reporting the use of TB assays to compute cell number and viability of 2D and 3D cultures. However, the literature still lacks studies regarding the reliability of the TB assay in terms of assessment of its repeatability and reproducibility. Methods: We compared the TB assay's measurements obtained by two biologists who analysed 105 different samples in double-blind for a total of 210 counts performed. We measured: (a) the repeatability of the count performed by the same operator; (b) the reproducibility of counts performed by the two operators. Results: There were no significant differences in the results obtained with 2D and 3D cell cultures: we estimated an approximate variability of 5% when the TB assay was used to assess the viability of the culture, and a variability of around 20% when it was used to determine the cell population density. Conclusions: The main aim of this study was to make researchers aware of potential measurement errors when TB is used with a haemocytometer for counting and viability measurements in 2D and 3D cultures. -

Comparison of Count Reproducibility, Accuracy, and Time to Results
Comparison of Count Reproducibility, Accuracy, and Time to Results between a Hemocytometer and the TC20™ Automated Cell Counter Tech Frank Hsiung, Tom McCollum, Eli Hefner, and Teresa Rubio Note Bio-Rad Laboratories, Inc., Hercules, CA 94547 USA Cell Counting Bulletin 6003 Introduction Bead counts were performed by sequentially loading For over 100 years the hemocytometer has been used by and counting the same chamber of a Bright-Line glass cell biologists to quantitate cells. It was first developed for the hemocytometer (Hausser Scientific). This was repeated ten quantitation of blood cells but became a popular and effective times. The number of beads was recorded for all nine tool for counting a variety of cell types, particles, and even 1 x 1 mm grids. small organisms. Currently, hemocytometers, armed with Flow Cytometry improved Neubauer grids, are a mainstay of cell biology labs. Flow cytometry was performed using a BD FACSCalibur flow Despite its longevity and versatility, hemocytometer counting cytometer (BD Biosciences) and CountBright counting beads suffers from a variety of shortcomings. These shortcomings (Life Technologies Corporation). Medium containing 50,000 include, but are not limited to, a lack of statistical robustness at CountBright beads was combined one-to-one with 250 µl low sample concentration, poor counts due to device misuse, of cells in suspension, yielding a final solution containing and subjectivity of counts among users, in addition to a time- 100 beads/µl. This solution was run through the flow consuming and tedious operation. In recent years automated cytometer until 10,000 events were collected in the gate cell counting has become an attractive alternative to manual previously defined as appropriate for non-doublet beads in hemocytometer–based cell counting, offering more reliable the FSC x SSC channel. -

Antimicrobial Effect of Zophobas Morio Hemolymph Against Bovine
microorganisms Article Antimicrobial Effect of Zophobas morio Hemolymph against Bovine Mastitis Pathogens Mengze Du y, Xiaodan Liu y, Jiajia Xu , Shuxian Li, Shenghua Wang, Yaohong Zhu and Jiufeng Wang * Department of Veterinary Clinical Sciences, College of Veterinary Medicine, China Agricultural University, Beijing 100193, China; [email protected] (M.D.); [email protected] (X.L.); [email protected] (J.X.); [email protected] (S.L.); [email protected] (S.W.); [email protected] (Y.Z.) * Correspondence: [email protected]; Tel.: +86-1355-221-6698 These authors contributed equally to this work. y Received: 2 September 2020; Accepted: 25 September 2020; Published: 28 September 2020 Abstract: Coliforms and Staphylococcus spp. infections are the leading causes of bovine mastitis. Despite extensive research and development in antibiotics, they have remained inadequately effective in treating bovine mastitis induced by multiple pathogen infection. In the present study, we showed the protective effect of Zophobas morio (Z. morio) hemolymph on bovine mammary epithelial cells against bacterial infection. Z. morio hemolymph directly kills both Gram-positive and Gram-negative bacteria through membrane permeation and prevents the adhesion of E. coli or the clinically isolated S. simulans strain to bovine mammary epithelial (MAC-T) cells. In addition, Z. morio hemolymph downregulates the expression of nucleotide-binding oligomerization domain (NOD)-like receptor family member pyrin domain-containing protein 3 (NLRP3), caspase-1, and NLRP6, as well as inhibits the secretion of interleukin-1β (IL-1β) and IL-18, which attenuates E. coli or S. simulans-induced pyroptosis. Overall, our results suggest the potential role of Z. morio hemolymph as a novel therapeutic candidate for bovine mastitis. -

Medical Laboratory Assistant, a Suggested Guide for A
p .7/ tWI .7- y,1.. P O R T R ESUM ED 013 321 VT 002 2L4 MEDICAL LABORATORY ASSISTANT, A SUGGESTEDGUIDE FOR A TRAINING PROGRAM. OFFICE OF EDUCATION, WASHINGTON, D.C. REPORT NUMBER 0E-87017 PUB DATE 66 EDRS PRICE MF-$0.50 HC-$4.92 123P. DESCRIPTORS-. *MEDICAL LABORATORY ASSISTANTS,TEACHING GUIDES, *PROGRAM PLANNING, PROGRAM DEVELOPMENT,*CURRICULUM GUIDES, CURRICULUM, *HEALTH OCCUPATIONSEDUCATION, POST SECONDARY EDUCATION, MDTA PROGRAMS, INFORMATION IS GIVEN TO ASSIST IN ORGANIZINGAND ADMINISTERING A TRAINING PROGRAM FOR MEDICAL LABORATORY ASSISTANTS IN A VARIETY OF SETTINGS AND TO PROVIDEGUIDANCE IN ESTABLISHING NEW PROGRAMS AND IN EVALUATINGEXISTING ONES. THE MATERIAL WAS PREPARED UNDER THE DIRECTIONOF THE NATIONAL COMMITTEE =OR CAREERS IN MEDICAL TECHNOLOGY.PATHOLOGISTS AND MEDICAL TECHNOLOGISTS PARTICIPATEDIN THE ORGANIZATIONAL AND DEVELOPMENTAL STAGES. ALL MATERIAL WAS REVIEWEDBY A REPRESENTATIVE NATIONAL GROUP OF EXPERTCONSULTANTS IN THE FIELD OF LABORATORY MEDICINE. THE 12-MONTH PROGRAM WAS DESIGNED FOR HIGH SCHOOL GRADUATES OR THEIREQUIVALENT TO SE ADMINISTERED BY A TEACHING STAFF COMPOSEDOF A NATIONAL DIRECTOR, A. TEACHING SUPERVISOR, ANDINSTRUCTORS. AN OUTLINE OF INFORMATIONAL MATERIAL TO BE PRESENTEDIN THE CLASSROOM, LABORATORY PROCEDURES TO BE DEMONSTRATEDAND THEN PERFORMED AS DIRECT EXERCISES BY THE STUDENTS, AS WELLAS RELEVANT BIBLIOGRAPHIES, AUDIOVISUAL AIDS, AND STUDYQUESTIONS ARE PRESENTED FOR THE FOLLOWING UNITS (1) ORIENTATION TO THE CLINICAL LABORATORY,(2) BACTERIOLOGY,(3) SEROLOGY, (4) PARASITOLOGY,(5) HEMATOLOGY, (6) CLINICAL CHEMISTRY,(7) BLOOD BANKING,(8) ROUTINE ANALYSIS, AND (9) BASAL METABOLISM -- ELECTROCARDIOGRAPHY. THIS DOCUMENT IS AVAILABLE AS GPO NUMBER FS 5.267-07017 FOR 60 CENTS FROMSUPERINTENDENT OF DOCUMENTS, U.S. GOVERNMENT PRINTING OFFICE,WASHINGTON, D.C. 20402. (PS) cir 02281"2=10. -

The Complete Blood Count Sample Report 1
THE COMPLETE BLOOD COUNT SAMPLE REPORT 1. Name and address of the lab where the test was performed. Tests may be run in a physician office lab, a lab located in a Different laboratories generate reports that can vary greatly in appearance and in the order and kind of The Complete Blood Count Sample Report clinic or hospital, and/or samples may be sent to a reference information included. This is one example of what a lab report for a Complete Blood Count may look like. laboratory for analysis. NamesDifferent and laboratories places used generate have been reports made that up can for vary illustrative greatly inpurposes appearance only. andThe innumbered the order keyand tokind the of right 2. Date this copy of the report was printed. This date may be explainsinformation a few included. of the reportThis is elements.one exampl e of what a lab report for a Complete Blood Count may look like. different than the date the results were generated, especially Names and places used have been made up for illustrative purposes only. Point your cursor at a number on cumulative reports (those that include results of several to learn about the different report elements. different tests run on different days). 3. Patient name or identifier. Links results to the correct person. 1 University Medical Center, Dept. of Pathology Report Date/Time: 123 University Way, City, ST 12345 02/10/2014 16:40 2 4. Patient identifier and identification number. Links results to the correct person. 3 Name: Doe, John Q. Age/Sex: 73/M DOB: 01/01/1941 5. -

Evaluation of Complete Blood Count Parameters for Diagnosis In
Original Investigation / Özgün Araştırma DOI: 10.5578/ced.68886 • J Pediatr Inf 2020;14(2):e55-e62 Evaluation of Complete Blood Count Parameters for Diagnosis in Children with Sepsis in the Pediatric Intensive Care Unit Pediatrik Yoğun Bakım Ünitesinde Sepsisli Çocuklarda Tanı İçin Tam Kan Sayımı Parametrelerinin Değerlendirilmesi Fatih Aygün1(İD), Cansu Durak1(İD), Fatih Varol1(İD), Haluk Çokuğraş2(İD), Yıldız Camcıoğlu2(İD), Halit Çam1(İD) 1 Department of Pediatric Intensive Care, Istanbul University School of Cerrahpasa Medicine, Istanbul, Turkey 2 Department of Pediatric Infectious Diseases, Istanbul University School of Cerrahpasa Medicine, Istanbul, Turkey Cite this article as: Aygün F, Durak C, Varol F, Çokuğraş H, Camcıoğlu Y, Çam H. Evaluation of complete blood count parameters for diagnosis in children with sepsis in the pediatric intensive care unit. J Pediatr Inf 2020;14(2):e55-e62. Abstract Öz Objective: Early diagnosis of sepsis is important for effective treatment Giriş: Erken tanı sepsiste etkili tedavi ve iyi prognoz için önemlidir. C-re- and improved prognosis. C-reactive protein (CRP) and procalcitonin aktif protein (CRP) ve prokalsitonin (PKT) sepsiste en sık kullanılan bi- (PCT) are the most commonly used biomarkers for sepsis. However, their yobelirteçlerdir. Fakat rutinde kullanımı maliyet etkin değildir. Tam kan routine usage is not cost-effective. Complete Blood Count (CBC) param- sayımı (TKS) parametrelerinden eritrosit dağılım genişliği (EDG), nötrofil eters including red cell distribution width (RDW), neutrophil to lympho- lenfosit oranı (NLO), trombosit lenfosit oranı (TLO), ortalama trombosit cyte ratios (NLR), platelet to lymphocyte ratios (PLR), mean platelet vol- hacmi (OTH) basit ve kolay olarak hesaplanmaktadır. Bu çalışmanın ama- ume (MPV), and hemoglobin are simple and easily calculated. -

A Method for High Throughput Determination
Hazan et al. BMC Microbiology 2012, 12:259 http://www.biomedcentral.com/1471-2180/12/259 METHODOLOGY ARTICLE Open Access A method for high throughput determination of viable bacteria cell counts in 96-well plates Ronen Hazan1,2,3,4†, Yok-Ai Que1,2,3†, Damien Maura1,2,3 and Laurence G Rahme1,2,3* Abstract Background: There are several methods for quantitating bacterial cells, each with advantages and disadvantages. The most common method is bacterial plating, which has the advantage of allowing live cell assessment through colony forming unit (CFU) counts but is not well suited for high throughput screening (HTS). On the other hand, spectrophotometry is adaptable to HTS applications but does not differentiate between dead and living bacteria and has low sensitivity. Results: Here, we report a bacterial cell counting method termed Start Growth Time (SGT) that allows rapid and serial quantification of the absolute or relative number of live cells in a bacterial culture in a high throughput manner. We combined the methodology of quantitative polymerase chain reaction (qPCR) calculations with a previously described qualitative method of bacterial growth determination to develop an improved quantitative method. We show that SGT detects only live bacteria and is sensitive enough to differentiate between 40 and 400 cells/mL. SGT is based on the re-growth time required by a growing cell culture to reach a threshold, and the notion that this time is proportional to the number of cells in the initial inoculum. We show several applications of SGT, including assessment of antibiotic effects on cell viability and determination of an antibiotic tolerant subpopulation fraction within a cell population. -
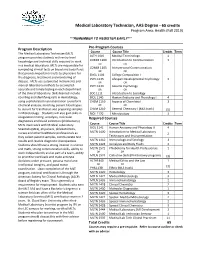
Medical Laboratory Technician 65 Credit AAS Program Guide
Medical Laboratory Technician, AAS Degree - 65 credits Program Area: Health (Fall 2019) ***REMEMBER TO REGISTER EARLY*** Program Description Pre-Program Courses Course Course Title Credits Term The Medical Laboratory Technician (MLT) program provides students with entry level ALTH 1410 Medical Terminology 1 knowledge and technical skills required to work COMM 1100 Introduction to Communication 3 in a medical laboratory. MLTs are responsible for OR OR COMM 1105 Interpersonal Communication completing clinical tests on blood and body fluids OR OR that provide important results to physicians for ENGL 1106 College Composition I the diagnosis, treatment and monitoring of PSYC 1135 Lifespan Developmental Psychology 3 disease. MLTs use automated instruments and OR OR manual laboratory methods to accomplish PSYC 1120 General Psychology accurate and timely testing in each department OR OR of the clinical laboratory. Skills learned include SOC 1111 Introduction to Sociology counting and identifying cells in Hematology, BIOL 1140 Human Anatomy and Physiology I 4 using sophisticated instrumentation to perform CHEM 1110 Aspects of Chemistry I 3 chemical analysis, matching patient blood types OR OR to donors for transfusion and preparing samples CHEM 1210 General Chemistry I (MLS track) (5) in Microbiology. Students will also gain skills in BIOL 1170 Microbiology 3 coagulation testing, urinalysis, molecular Required Courses diagnostics and blood collection (phlebotomy). MLTs must work with Medical Laboratory Course Course Title Credits Term Scientists (MLS), physicians, phlebotomists, BIOL 1141 Human Anatomy and Physiology II 4 MLTN 1400 Introduction to Medical Laboratory nurses and other healthcare professionals as 2 they collect patient samples, communicate test Techniques and Instrumentation results and resolve diagnostic discrepancies. -

Absolute Measurements of Cerebral Perfusion and Oxygenation in Rats with Near-Infrared Spectroscopy Bertan Hallacoglu1, Angelo Sassaroli1, Irwin H
Absolute measurements of cerebral perfusion and oxygenation in rats with near-infrared spectroscopy Bertan Hallacoglu1, Angelo Sassaroli1, Irwin H. Rosenberg2, Aron Troen2 and Sergio Fantini1 Tufts University Department of Biomedical Engineering, Medford, MA (1) and USDA Human Nutrition Research Center on Aging, Boston, MA (2) Brain microvascular pathology is a common finding in Alzheimer’s disease and other dementias. However, Figure 2 – Time traces of [Hb], [HbO2], [HbT], StO2 and SaO2 during the hypoxia and hypercapnia protocols 10 and 20 weeks after Figure 3 – Illustration of the differences in animal groups and the extent to which microvascular abnormalities cause or contribute to cognitive impairment is unclear. the start of folate deficient diet (blue and red lines represent the mean values for each dietary group, whereas, dashed lines indicate changes within each group between weeks 10 and 20 in Dietary vascular risk factors, including poor folate status are potentially modifiable predictors of cognitive the range corresponding to ± one standard error from the mean). A first striking result is the consistency of baseline values across cerebral tissue saturation (StO2) and concentration of impairment among older adults. Folate deficiency in rat impairs cognition and causes cerebral animals within a group (control or folate deficient), and the reproducibility of baseline values measured at weeks 10 and 20. hemoglobin ([HbT]), blood concentration of hemoglobin ([HbT]b), microvascular damage, without concomitant neurodegeneration [1]. We hypothesized that folate Absolute brain total hemoglobin concentration ([HbT]) and tissue oxygen saturation (StO2) are significantly reduced by folate and partial blood volume (Vb/Vt) Eq. (1). FD rats have deficiency might result in functional decrements in cerebral oxygen delivery and vascular reactivity.