Urate in Fingernail Represents the Deposition of Urate Burden in Gout
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
![Directors and Parties Involved in the [Redacted]](https://docslib.b-cdn.net/cover/9348/directors-and-parties-involved-in-the-redacted-189348.webp)
Directors and Parties Involved in the [Redacted]
THIS DOCUMENT IS IN DRAFT FORM, INCOMPLETE AND SUBJECT TO CHANGE AND THAT INFORMATION MUST BE READ IN CONJUNCTION WITH THE SECTION HEADED “WARNING” ON THE COVER OF THIS DOCUMENT. DIRECTORS AND PARTIES INVOLVED IN THE [REDACTED] DIRECTORS Name Address Nationality Chairman and executive Director Dr. Yiyou CHEN (陳一友) 5-1604, No. 201 Jianghan American East Road Binjiang District Hangzhou, Zhejiang PRC Executive Director Mr. Yeqing ZHU (朱葉青) 5-702, North District of Chinese Ruyuan, Xibeiwang Town Haidian District Beijing PRC Non-executive Directors Mr. Naxin YAO (姚納新) Room 2802, Unit 2 Chinese Building 2 Shuijing Lanxuan Area Binjiang District Hangzhou PRC Ms. Nisa Bernice Wing-Yu 1/F, 15 Wang Chiu Road Chinese LEUNG, J.P. (梁頴宇) Kowloon (Hong Kong) Hong Kong Mr. Quan ZHOU (周瑔) 5-1-801, Yuanyang Fengjing Area Chinese Deshengmen Haidian District Beijing PRC Mr. Siu Wai NG (伍兆威) Flat D, 16/F Chinese Block 6, Sorrento (Hong Kong) 1 Austin Road West Kowloon, Hong Kong – 162 – THIS DOCUMENT IS IN DRAFT FORM, INCOMPLETE AND SUBJECT TO CHANGE AND THAT INFORMATION MUST BE READ IN CONJUNCTION WITH THE SECTION HEADED “WARNING” ON THE COVER OF THIS DOCUMENT. DIRECTORS AND PARTIES INVOLVED IN THE [REDACTED] Name Address Nationality Independent non-executive Directors Mr. Danke YU (余丹柯) 34 Belinda Crescent Australian Wheelers Hill Victoria State Australia Prof. Hong WU (吳虹) No. 607, Building 1 Chinese Wudaokou Jiayuan No. 3 Zhanchunyuan West Road Haidian District Beijing PRC Dr. Kwok Tung LI, Donald, 4th Floor, Block K, Pine Court Chinese S.B.S., J.P. (李國棟) 5 Old Peak Road (Hong Kong) Mid-levels Hong Kong Please see the section headed “Directors and Senior Management” in this Document for further details of our Directors. -
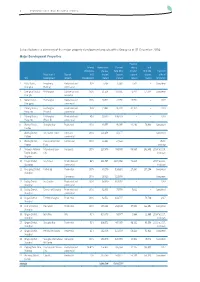
Major Development Properties
1 SHANGHAI INDUSTRIAL HOLDINGS LIMITED Set out below is a summary of the major property development projects of the Group as at 31 December 2016: Major Development Properties Pre-sold Interest Approximate Planned during Total attributable site area total GFA the year GFA sold Expected Projects of SI Type of to SI (square (square (square (square date of City Development property Development meters) meters) meters) meters) completion 1 Kaifu District, Fengsheng Residential and 90% 5,468 70,566 7,542 – Completed Changsha Building commercial 2 Chenghua District, Hi-Shanghai Commercial and 100% 61,506 254,885 75,441 151,644 Completed Chengdu residential 3 Beibei District, Hi-Shanghai Residential and 100% 30,845 74,935 20,092 – 2019 Chongqing commercial 4 Yuhang District, Hi-Shanghai Residential and 85% 74,864 230,484 81,104 – 2019 Hangzhou (Phase I) commercial 5 Yuhang District, Hi-Shanghai Residential and 85% 59,640 198,203 – – 2019 Hangzhou (Phase II) commercial 6 Wuxing District, Shanghai Bay Residential 100% 85,555 96,085 42,236 76,966 Completed Huzhou 7 Wuxing District, SIIC Garden Hotel Hotel and 100% 116,458 47,177 – – Completed Huzhou commercial 8 Wuxing District, Hurun Commercial Commercial 100% 13,661 27,322 – – Under Huzhou Plaza planning 9 Shilaoren National International Beer Composite 100% 227,675 783,500 58,387 262,459 2014 to 2018, Tourist Resort, City in phases Qingdao 10 Fengze District, Sea Palace Residential and 49% 381,795 1,670,032 71,225 – 2017 to 2021, Quanzhou commercial in phases 11 Changning District, United 88 Residential -

Report on the Parliamentary Trade Mission to Shanghai Honourable
Report on the Parliamentary Trade Mission to Shanghai Honourable Curtis Pitt MP Speaker of the Legislative Assembly 21 -27 September 2019 1 TABLE OF CONTENTS EXECUTIVE SUMMARY ................................................................................... 3 OBJECTIVES OF THE QUEENSLAND PARLIAMENTARY TRADE DELEGATION ..... 4 QUEENSLAND – CHINA RELATIONSHIP ........................................................... 5 MISSION DELEGATION MEMBERS .................................................................. 9 PROGRAM ................................................................................................... 10 RECPEPTION: QUEENSLAND YOUTH ORCHESTRA ENSEMBLE PERFORMANCE AND DINNER WITH QUEENSLAND DELEGATES ............................................. 21 MEETING: BUNDABERG BREWED DRINKS .................................................... 23 MEETING: AUSTCHAM SHANGHAI ............................................................... 25 MEETING: SHANGHAI PEOPLE’S CONGRESS ................................................. 27 SITE VISIT: SENSETIME ................................................................................. 29 RECEPTION: QUEENSLAND GOVERNMENT RECEPTION ................................ 32 MEETING: ALIBABA GROUP .......................................................................... 34 TIQ BUSINESS DINNER ................................................................................. 40 MEETING: JINSHAN DISTRICT PEOPLE’S CONGRESS ...................................... 41 SITE VISIT: FENGJING ANCIENT TOWN, -
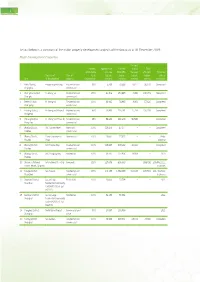
Set out Below Is a Summary of the Major Property Development Projects of the Group As at 31 December 2019: Major Development
1 Set out below is a summary of the major property development projects of the Group as at 31 December 2019: Major Development Properties Pre-sold Interest Approximate Planned during Total attributable site area total GFA the year GFA sold Expected Projects of Type of to SI (square (square (square (square date of City SI Development property Development meters) meters) meters) meters) completion 1 Kaifu District, Fengsheng Building Residential and 90% 5,468 70,566 6,627 30,870 Completed Changsha commercial 2 Chenghua District, Hi-Shanghai Residential and 100% 61,506 254,885 4,996 190,153 Completed Chengdu commercial 3 Beibei District, Hi-Shanghai Residential and 100% 30,845 74,935 3,301 57,626 Completed Chongqing commercial 4 Yuhang District, Hi-Shanghai (Phase I) Residential and 85% 74,864 230,484 27,758 150,289 Completed Hangzhou commercial 5 Yuhang District, Hi-Shanghai (Phase II) Residential and 85% 59,640 198,203 56,539 – Completed Hangzhou commercial 6 Wuxing District, SIIC Garden Hotel Hotel and 100% 116,458 47,177 – – Completed Huzhou commercial 7 Wuxing District, Hurun Commercial Commercial 100% 13,661 27,322 – – Under Huzhou Plaza planning 8 Wuxing District, SIIC Tianlan Bay Residential and 100% 115,647 193,292 26,042 – Completed Huzhou commercial 9 Wuxing District, SIIC Yungjing Bay Residential 100% 68,471 207,906 28,953 – 2020 Huzhou 10 Shilaoren National International Beer City Composite 100% 227,675 806,339 – 333,798 2014 to 2022, Tourist Resort, Qingdao in phases 11 Fengze District, Sea Palace Residential and 100% 170,133 -

World Bank Document
RP707 Puxi Section Project of Southern Transportation Trunk V3 Perfecting Project of Bailonggang Area, Shanghai ——Funded by World Bank Public Disclosure Authorized Southern Transportation Trunk Perfecting Project for Sewage Treatment in Bailonggang Area, Shanghai Puxi Section Project Resettlement Action Plan Public Disclosure Authorized Public Disclosure Authorized Shanghai Urban Drainage Corporation August2008 Public Disclosure Authorized 1 Endorsement Letter for the Resettlement Plan Shanghai Municipal Government has applied for a loan from WB to finance Shanghai Southern Transportation Trunk Perfecting Project for Sewage Treatment Bailonggang Area. Therefore, the Project must be implemented in compliance with ADB safeguard requirements. This Resettlement Plan is prepared in accordance with the laws of the PRC, Shanghai Municipality, as well as WB’s Policy on Involuntary Resettlement and it constitutes the basis for land acquisition, house demolition and resettlement of the project. Shanghai Municipal Government hereby confirms the content of this Resettlement Plan and will guarantee the land acquisition, compensation and relocation budget being provided according to the provisions of this Resettlement Plan. This Resettlement Plan is based on the feasibility study report and the initial surveys. If the final implemented components are different from the contents described in the feasibility study report and that will cause the substantial impact on the Resettlement Plan, this Resettlement Plan should be future modified and approved by WB before its implementation. Shanghai Municipal People’s Government __________ (Signature) _____________ (Date) 2 Preface I. The objective of Resettlement Action Plan 1 This Resettlement Action Plan is formulated on the basis of the laws, regulations and policies of the People’s Republic of China, and Local Government as well as the Guidelines of the World Bank (Operational Directive for Involuntary Resettlement OP 4.12). -

Mainland China
SUPPLIER’S GUIDE TO INVOICING — ACCENTURE MAINLAND CHINA 1.1 Contents 1.1 Contents ..................................................................................................................... 2 2 Introduction ................................................................................................................. 3 2.1 Establishing and Maintaining Your Accenture Supplier Profile .................................... 3 2.2 Payment Terms .......................................................................................................... 3 2.3 Preparing Invoices for Accenture ................................................................................ 3 2.4 Special Instructions for Purchase Order (PO) Invoices ............................................... 4 2.5 Submitting Invoices to Accenture................................................................................ 4 2.5.1 Other Systems Used for Invoice Submissions ............................................................ 5 2.6 Further Information & Assistance................................................................................ 5 2.7 Invoice to Addresses .................................................................................................. 6 3 Document Version Control ......................................................................................... 9 pg. 2 2 Introduction Dear Supplier, thank you for providing goods or services to Accenture. Accenture always aims to pay suppliers on time, within the payment terms that have -
Apartments the Shanghai Guide 2016 * Serviced Apartments
The Shanghai Guide 2016 * Serviced Apartments The Shanghai Guide 2016 * Serviced Apartments 2016 The Shanghai Guide Serviced Apartments Reader's Choice Award Choice Reader's Shanghai Centre Serviced Apartments 172 | The Shanghai Guide www.cityweekend.com.cn The Shanghai Guide | 173 The Shanghai Guide 2016 * Serviced Apartments The Shanghai Guide 2016 * Serviced Apartments Arcadia Ascott Heng Shan Shanghai Central Residences II Grand Gateway 66 Premier luxury residences Work, live and play in Xuhui Experience a green retreat Serviced Apartments Developed by Sun Hung Kai Properties, this massive Nothing says “city sanctuary” more than a low-rise, Located on Huashan Lu in a charming tree-lined Services catered to your lifestyle property estate covers 1,600 sq. meters, including a secluded villa, right in the heart of the action. Char- area, these upscale residences boast proximity to Grand Gateway 66 offers convenient, luxury living in green belt of 400 sq. meters and a large clubhouse full acterized by tree-lined streets and a never-ending cultural and architectural landmarks in addition to one of the city’s most popular commercial shopping of indoor and outdoor recreational activities. Arcadia is array of bars and restaurants, Xuhui is one of Shang- modern amenities. As part of the Kerry Properties hubs. These fully furnished residences are situated comprised of three towers—the Grand Mayfair, Belgra- hai’s most popular districts to live and play, for locals group, which manages developments across Asia, directly above the Xujiahui Metro station, providing via and Parklane—each featuring private luxury resi- and expats alike. Conveniently situated right next Central Residences II offers their signature service direct access to Metro Lines 1 and 9. -

Business Incubator of Tongji University Science Park
1. To create a knowledge-based service innovation cluster by the radiation effect of the Tongji knowledge-based economic circle. 2. To boost the development of joint production and Business Incubator of Tongji research and construct a chain-wide innovation and start-up service system. University Science Park 3. To build a dream together with entrepreneurs by the concept of seamless collaboration team service. Location: Yangpu District, Shanghai General Introduction Address: Room 105, 65 Chifeng Road, Yangpu District, Shanghai Business Incubator of Tongji University Science Park Number of Incubated foreign invested enterprises, was established in December 2003, located in Tongji joint ventures or overseas talent startups: 30 University South Campus in Chifeng Road. It is the national Website: www.tj-ibi.com high-tech business service center and vice president unit of Major industries: Modern design, energy saving and Shanghai Science and Technology Incubator Association. environmental protection, electronic With more than 1,400 registered enterprises reside in information, rail transportation,etc. incubation sites, it covers an incubation area of 20,000 square meters. As the incubation platform of Tongji University National University Science and Technology Park, the company is Results list specialized in the incubation of small and medium enterprises of science and technology, providing the enterprises residence services and business incubation services. Performance List 55 enterprises in the park have been identified as high-tech enterprises, 9 identified as the Shanghai Little Giant Cultivating Enterprises, 4 as Shanghai Little Giant Enterprises, and 16 as Yangpu District Little Giant Enterprises. Resident enterprises have over 1000 intellectual property rights. There are 3 companies have been listed on the NEEQ and 1 company has been listed in the Shanghai Equity Trading Center. -

Correlation Analysis Housing Prices and Regions in Shanghai Jian Chena,* and Hai-Lan Panb No
2017 International Conference on Modern Education and Information Technology (MEIT 2017) ISBN: 978-1-60595-468-4 Correlation Analysis Housing Prices and Regions in Shanghai Jian CHENa,* and Hai-lan PANb No. 2360, Jinghai Road, Pudong District, Shanghai Polytechnic University, Shanghai, China [email protected], [email protected] *Corresponding author Keywords: Correlation Analysis, Housing Price, Shanghai, Relation Search Abstract. Most existing house price index construction methods are developed mainly based on transaction data from the secondary housing market, and are not necessarily suitable for the nascent housing markets where a predominant portion of housing transactions are in new units. We evaluate and compare the performances of three most common house price measurement methods in the newly-built housing sector, including the simple average method without quality adjustment, the matching approach with the repeat sales modeling framework, and the hedonic modeling approach. Introduction With the rapid economic development, the real estate industry has become a pillar industry of China's national economy. Excessive growth of house prices not only affects the standard of living, but also the entire national economy sustained. Steady and rapid development has brought instability RATES livelihood a hot topic. It has great significance on the price forecast. Housing problem can be seen as a high degree of complexity, uncertainty, nonlinear, dynamic, open complex giant system, which is not only affected by economic, political, but also by people's behavior, psychology, decision-making, and many other random non-quantifiable factors related. Shanghai real estate market supply and booming form a strong momentum, real estate development and sales hit record highs, the Shanghai housing market ushered in the "rebound." In contrast, levels of disposable income and purchasing power of urban residents did not follow "gone." Thus, prices of supply and demand have become the focus of mutual concern. -

China's Financial Hub Wants to Be Global AI Powerhouse
CHINA DAILY | HONG KONG EDITION Thursday, January 14, 2021 | 15 BUSINESSFOCUS Editor's Note: Shanghai’s economy remained resilient despite the COVID-19 impact. To further unleash its enormous development potential, Shanghai launched 64 major projects with a combined investment of 273.4 billion yuan ($42.2 billion) on the first working day of 2021, covering sectors like scientif- ic and technological innovation, infrastructure and people’s livelihoods. Here are some key projects identified for different fields. Shanghai looks to be biomed valley of Asia By ZHOU WENTING in Shanghai “Several innovative anti-tumor [email protected] drugs by the company are cur- rently at various clinical trial sta- Multiple new biomedicine pro- ges, and there is also an urgent jects were included on Jan 4 need for an industrialized plat- among the 64 new projects in form to ensure that the R&D pro- Shanghai, which is positioning grams will eventually be landed itself to be a biomedical hub for in Shanghai’s Zhangjiang,” the Asia. company added. The new projects have attract- It said that the base will not ed significant investment and only serve research and develop- will help construct a better indus- ment for its own purposes but trial layout throughout the city also provide a one-stop service and further elevate the develop- platform for small and medium- ment level and global market sized businesses specializing in competence of the biomedical biomedical R&D in Zhangjiang. industry chain, the city govern- It will also help attract major ment said. innovations to be commercial- They will also help gather high- ized in Shanghai to form regional quality resources from home and advantages. -

Dan Washburn in His Neighborhood, Hua Ye Xiao Qu. Dan Will Answer Questions About Shanghai on Tuesday, July 11, at Budgettravelo
Dan Washburn in his neighborhood, Hua Ye Xiao Qu. Dan will answer questions about Shanghai on Tuesday, July 11, at BudgetTravelOnline.com My Shanghai Is Better ThanYours Four years ago, Dan Washburn dropped everything, moving from suburban Georgia to China’s most dynamic city. As the editor of Shanghaiist.com, he’s since learned far more about Shanghai than we ever could hope to, so we’ve invited him to tell us the best places to eat, shop, and play. Photographs by Ryan Pyle I ARRIVED IN SHANGHAI BY ACCIDENT, REALLY. I had a decent that Shanghai is certainly never boring. The city is constantly gig at a small newspaper outside Atlanta—they paid me to try changing, always reinventing itself. The relentless pace of the a new activity each week (bull riding, skydiving, nude water place is addictive. I have fallen in love with Shanghai (and no, volleyball...) and write about my experiences. Not bad. Of course, that’s not hyperbole). I also had to cover high school tennis, but there are always trade- I had a personal website before I moved to Shanghai, so I kept offs in life. Nearly four years into my Georgia stay, with another it going once I got here. Hard to believe now, but back in 2002, high school sports season on the horizon, I suddenly decided I there was a dearth online of English-language information about needed to make a change. I didn’t know what, and I didn’t know the city. My blog, Shanghaidiaries.com, quickly attracted a dedi- where. -

內地網絡mainland Network
內地網絡 Mainland Network 南洋商業銀行(中國)有限公司 NANYANG COMMERCIAL BANK (CHINA), LIMITED Head Office Nanyang Commercial Bank Building, No.800 Century Avenue, (86-21) 3856 6666 總行 Pudong New Area, Shanghai, China (86-21) 6887 9800 上海市浦東新區世紀大道800號南洋商業銀行大廈 Branch Address Telephone/Fax 分行名稱 地址 電話號碼╱傳真號碼 The Mainland of China 中國內地 Shenzhen Branch L140-142, Tower 4, Excellence Century Center, Fuhua 3rd Road, (86-755) 8233 0230 深圳分行 Futian District, Shenzhen, China (86-755) 8234 4506 深圳市福田區福華三路卓越世紀中心4號樓L140-142商舖 Shenzhen Shekou Sub-Branch G/F, Finance Centre, No.22 Taizi Road, (86-755) 2682 8788 深圳蛇口支行 Shekou, Nanshan District, Shenzhen, China (86-755) 2669 2466 深圳市南山區蛇口太子路22號金融中心地下 Shenzhen Luohu Sub-Branch G/F, The Kwangtung Provincial Bank Building, (86-755) 2515 6333 深圳羅湖支行 No.1013 Ren Min Nan Road, Luohu District, Shenzhen, China (86-755) 8229 1717 深圳市羅湖區人民南路1013號廣東省銀行大廈一樓 Shenzhen Baoan Sub-Branch Unit 108 Xushida Garden, Xin An Si Road, (86-755) 2785 3302 深圳寶安支行 Baoan District 34-2, Shenzhen, China (86-755) 2785 3415 深圳市寶安區34-2區新安四路旭仕達名苑一層108號 Shenzhen Futian Sub-Branch 1/F, Shen Ye Garden Club House, Caitian Road, (86-755) 8294 2929 深圳福田支行 Futian District, Shenzhen, China (86-755) 8290 2016 深圳市福田區彩田路深業花園會所1樓 Shenzhen Dongguan No. C-112, C-204, Dingfeng International Plaza, (86-769) 2662 6888 Sub-Branch No.19 Dongguan Avenue, Dongcheng District, Dongguan, China (86-755) 2360 6321 深圳東莞支行 東莞市東城區東莞大道19號鼎峰國際廣場C-112、C-204號商舖 Shenzhen Jiabin Sub-Branch 1/F, Block C, Nanyang Mansion, No.2002 Jianshe Road, (86-755) 8220 9955 深圳嘉賓支行 Luohu District, Shenzhen,