Production of Dextrose and Maltose Syrups Using an Enzyme Derived from Rice
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Distribution of Carbohydrase Activities in the Small Intestine in Early Weaned Calves
The Distribution of Carbohydrase Activities in the Small Intestine in Early Weaned Calves Toshikazu MIYASHIGEand Shigefusa YAHATA Chugoku National Agricultural Experiment Station, Oda-shi 694-01 (Received December 8, 1980) Abstract 1. The carbohydrase activities of the small intestine of early weaned calves were compared with those of nursing ones at 26 weeks of age. 2. In the early weaned calves, the pH value of content of the caecum was observed to be lower than that of the nursing ones, suggesting that more soluble carbohydrates would have escaped from ruminal fermentation and reached the caecum. 3. The values of maltase and iso maltase activities of the early weaned calves were not different from those of the nursing ones. However, maltase activity of the early weaned calves distributed more constantly with relatively high level all over the small intestine. Lactase activity of the early weaned calves was almost the same as that of the nursing ones. Dextranase and amylase activities were also found in the small intestine of the early weaned calves. Jpn. J. Zootech. Sci., 52 (7): 532-536, 1981 In the previous study1) with nursing calves, it was shown that intestinal maltase activity was very low and did not increase with age. But the pattern of the distri- bution of maltase activity in the small intestine apparently changed with age and higher activity of maltase was found in the lower part of the small intestine at 26 weeks of age. Although the meaning of this change is not known, it is supposed that the change of the pattern would be correlated with the increase of solid food intake. -

Effect of a Multi-Carbohydrase and Phytase Complex on the Ileal And
animals Article Effect of a Multi-Carbohydrase and Phytase Complex on the Ileal and Total Tract Digestibility of Nutrients in Cannulated Growing Pigs 1, 2, 1, 1 1 3 Jia-Cheng Yang y, Li Wang y, Ya-Kuan Huang y, Lei Zhang , Rui Ma , Si Gao , Chang-Min Hu 4, Jlali Maamer 5, Cozannet Pierre 5, Aurélie Preynat 5, Xin Gen Lei 6 and Lv-Hui Sun 1,* 1 Department of Animal Nutrition and Feed Science, College of Animal Science and Technology, Huazhong Agricultural University, Wuhan 430070, China; [email protected] (J.-C.Y.); [email protected] (Y.-K.H.); [email protected] (L.Z.); [email protected] (R.M.) 2 Institute of Animal Science, Guangdong Academy of Agricultural Sciences, Guangzhou 510640, China; [email protected] 3 Demonstration Center of Hubei Province for Experimental Animal Science Education, Huazhong Agricultural University, Wuhan 430070, China; [email protected] 4 College of Veterinary Medicine, Huazhong Agricultural University, Wuhan 430070, China; [email protected] 5 Adisseo France S.A.S., Center of Expertise in Research and Nutrition, 03600 Commentry, France; [email protected] (J.M.); [email protected] (C.P.); [email protected] (A.P.) 6 Department of Animal Science, Cornell University, Ithaca, NY 14853, USA; [email protected] * Correspondence: [email protected]; Tel.: +86-130-0712-1983 These authors contributed equally to this work. y Received: 6 July 2020; Accepted: 14 August 2020; Published: 17 August 2020 Simple Summary: It is well accepted that monogastric livestock lack phytase in their gastrointestinal tracts. -

Exogenous Enzymes As Zootechnical Additives in Animal Feed: a Review
catalysts Review Exogenous Enzymes as Zootechnical Additives in Animal Feed: A Review Brianda Susana Velázquez-De Lucio 1, Edna María Hernández-Domínguez 1, Matilde Villa-García 1, Gerardo Díaz-Godínez 2, Virginia Mandujano-Gonzalez 3, Bethsua Mendoza-Mendoza 4 and Jorge Álvarez-Cervantes 1,* 1 Cuerpo Académico Manejo de Sistemas Agrobiotecnológicos Sustentables, Posgrado Biotecnología, Universidad Politécnica de Pachuca, Zempoala 43830, Hidalgo, Mexico; [email protected] (B.S.V.-D.L.); [email protected] (E.M.H.-D.); [email protected] (M.V.-G.) 2 Centro de Investigación en Ciencias Biológicas, Laboratorio de Biotecnología, Universidad Autónoma de Tlaxcala, Tlaxcala 90000, Tlaxcala, Mexico; [email protected] 3 Ingeniería en Biotecnología, Laboratorio Biotecnología, Universidad Tecnológica de Corregidora, Santiago de Querétaro 76900, Querétaro, Mexico; [email protected] 4 Instituto Tecnológico Superior del Oriente del Estado de Hidalgo, Apan 43900, Hidalgo, Mexico; [email protected] * Correspondence: [email protected]; Tel.: +52-771-5477510 Abstract: Enzymes are widely used in the food industry. Their use as a supplement to the raw Citation: Velázquez-De Lucio, B.S.; material for animal feed is a current research topic. Although there are several studies on the Hernández-Domínguez, E.M.; application of enzyme additives in the animal feed industry, it is necessary to search for new Villa-García, M.; Díaz-Godínez, G.; enzymes, as well as to utilize bioinformatics tools for the design of specific enzymes that work in Mandujano-Gonzalez, V.; certain environmental conditions and substrates. This will allow the improvement of the productive Mendoza-Mendoza, B.; Álvarez-Cervantes, J. -

Collection of Information on Enzymes a Great Deal of Additional Information on the European Union Is Available on the Internet
European Commission Collection of information on enzymes A great deal of additional information on the European Union is available on the Internet. It can be accessed through the Europa server (http://europa.eu.int). Luxembourg: Office for Official Publications of the European Communities, 2002 ISBN 92-894-4218-2 © European Communities, 2002 Reproduction is authorised provided the source is acknowledged. Final Report „Collection of Information on Enzymes“ Contract No B4-3040/2000/278245/MAR/E2 in co-operation between the Federal Environment Agency Austria Spittelauer Lände 5, A-1090 Vienna, http://www.ubavie.gv.at and the Inter-University Research Center for Technology, Work and Culture (IFF/IFZ) Schlögelgasse 2, A-8010 Graz, http://www.ifz.tu-graz.ac.at PROJECT TEAM (VIENNA / GRAZ) Werner Aberer c Maria Hahn a Manfred Klade b Uli Seebacher b Armin Spök (Co-ordinator Graz) b Karoline Wallner a Helmut Witzani (Co-ordinator Vienna) a a Austrian Federal Environmental Agency (UBA), Vienna b Inter-University Research Center for Technology, Work, and Culture - IFF/IFZ, Graz c University of Graz, Department of Dermatology, Division of Environmental Dermatology, Graz Executive Summary 5 EXECUTIVE SUMMARY Technical Aspects of Enzymes (Chapter 3) Application of enzymes (Section 3.2) Enzymes are applied in various areas of application, the most important ones are technical use, manufacturing of food and feedstuff, cosmetics, medicinal products and as tools for re- search and development. Enzymatic processes - usually carried out under mild conditions - are often replacing steps in traditional chemical processes which were carried out under harsh industrial environments (temperature, pressures, pH, chemicals). Technical enzymes are applied in detergents, for pulp and paper applications, in textile manufacturing, leather industry, for fuel production and for the production of pharmaceuticals and chiral substances in the chemical industry. -

Biochemical Characterization of Digestive Carbohydrases in the Rose Sawfly, Arge Rosae Linnaeus (Hymenoptera: Argidae)
J. Crop Prot. 2013, 2 (3): 305-318 ______________________________________________________ Biochemical characterization of digestive carbohydrases in the rose sawfly, Arge rosae Linnaeus (Hymenoptera: Argidae) Moloud Gholamzadeh Chitgar1, Seyed Mohammad Ahsaei2, Mohammad Ghadamyari1*, Mahbobe Sharifi1, Vahid Hosseini Naveh2 and Hadi Sheikhnejad1 1. Department of Plant Protection, Faculty of Agricultural Science, University of Guilan, Rasht, Iran. 2. Department of Plant protection, Faculty of Agricultural Sciences & Engineering, College of Agriculture and Natural Resources, University of Tehran, Karaj, Iran. Abstract: The rose sawfly, Arge rosae Linnaeus, is one of the most destructive pests of rose bushes in the north of Iran. Nowadays, many attempts have been made to reduce pesticide application by looking for new methods of pest control. A non chemical method for controlling insect pests including A. rosae can be achieved by using genetically engineered plants expressing carbohydrase inhibitors. Therefore, in present study we characterized biochemical properties of digestive carbohydrases in the gut of A. rosae for achieving a new method for control of this pest. The specific activity of α-amylase in the digestive system of last larval instars of A. rosae was obtained as 9.46 ± 0.06 μmol min-1 mg-1 protein. Also, the optimal pH and temperature for α-amylase were found to be at pH 8 and 50 °C. As calculated from Lineweaver-Burk plots, the Km and Vmax values for α-amylase were 0.82 mg/ml and 7.32 µmol min-1 mg-1 protein, respectively, when starch was used as substrate. The effects of ions on amylolytic activity showed that Mg2+ and Na+ significantly increased amylase activity, whereas SDS and EDTA decreased the enzyme activity. -

The Mechanism of Carbohydrase Action 5
246 Biochem. J. (1960) 76, 246 The Mechanism of Carbohydrase Action 5. ACTION OF HUMAN SALIVARY a-AMYLASE ON AMYLOPECTIN AND GLYCOGEN* By P. J. P. ROBERTSt AND W. J. W-HELANt Chemistry Department, University College of North Wales, Bangor (Received 11 January 1960) When human salivary oc-amylase acts on whole if any, esterified phosphorus (Schoch, 1953). It was pre- starch (amylose+amylopectin) there is produced pared from hand-sorted single-cross Tapicorn seed, (Bear a mixture of fermentable and unfermentable Hybrid Co., Decatur, Illinois, U.S.A.) as by Schoch (1957). sugars (Myrback, 1948). The fermentable sugars Buffers. These were 0-2 m-sodium acetate-acetic acid consist of maltose, maltotriose and/or glucose (see (pH 7.0) and 0-2M-sodiunm citrate-citric acid (pH 7-0). below). The non-fermentable sugars (a-limit Methods dextrins) are a mixture of higher oigosaccharides. Digests. Waxy-maize starch was moistened with ethanol Since the amylose component of starch, which and dissolved in 0-6N-sodium hydroxide by heating in a consists essentially only of 1:4-linked a-glucose boiling-water bath for 5 min. After cooling, the alkali was units, can be converted by the amylase completely neutralized to phenolphthalein either with sulphuric acid into maltose and maltotriose (Whelan & Roberts, [digests (1) and (2), see below] or hydrochloric acid [digest 1953) the a-limit dextrins must arise from the (3)]. Glycogen and the enzymes were dissolved in water. amylopectin component and may be assumed to After the substrate had dissolved, the buffer was added and contain the a-1:6-bonds which constitute the points then the enzyme. -

Glycoside Hydrolases Degrade Polymicrobial Bacterial Biofilms In
CORE Metadata, citation and similar papers at core.ac.uk Provided by Texas A&M Repository EXPERIMENTAL THERAPEUTICS crossm Glycoside Hydrolases Degrade Polymicrobial Bacterial Biofilms in Wounds Downloaded from Derek Fleming,a,b Laura Chahin,a* Kendra Rumbaugha,b,c Departments of Surgerya and Immunology and Molecular Microbiologyb and TTUHSC Surgery Burn Center of Research Excellence,c Texas Tech University Health Sciences Center, Lubbock, Texas, USA ABSTRACT The persistent nature of chronic wounds leaves them highly susceptible to invasion by a variety of pathogens that have the ability to construct an extracel- Received 15 September 2016 Returned for lular polymeric substance (EPS). This EPS makes the bacterial population, or biofilm, modification 15 October 2016 Accepted 15 November 2016 up to 1,000-fold more antibiotic tolerant than planktonic cells and makes wound Accepted manuscript posted online 21 healing extremely difficult. Thus, compounds which have the ability to degrade bio- November 2016 http://aac.asm.org/ films, but not host tissue components, are highly sought after for clinical applica- Citation Fleming D, Chahin L, Rumbaugh K. tions. In this study, we examined the efficacy of two glycoside hydrolases, ␣-amylase 2017. Glycoside hydrolases degrade polymicrobial bacterial biofilms in wounds. and cellulase, which break down complex polysaccharides, to effectively disrupt Antimicrob Agents Chemother 61:e01998-16. Staphylococcus aureus and Pseudomonas aeruginosa monoculture and coculture bio- https://doi.org/10.1128/AAC.01998-16. films. We hypothesized that glycoside hydrolase therapy would significantly reduce Copyright © 2017 American Society for EPS biomass and convert bacteria to their planktonic state, leaving them more sus- Microbiology. -

The Physiology and Biochemistry of the Digestion System of Termites from the Genus Anacanthotermes Jacobson, 1904
International Journal of Biology; Vol. 11, No. 4; 2019 ISSN 1916-9671 E-ISSN 1916-968X Published by Canadian Center of Science and Education The Physiology and Biochemistry of the Digestion System of Termites from the Genus Anacanthotermes Jacobson, 1904 Ikram I. Abdullaev1, Manzura B. Doschanova1, Zafar Sh. Matyakubov2, Abdulla I. Iskandarov2, & Feruza R. Rakhimbaeva2 1 Khorezm Academy of Mamun, Khiva, Uzbekistan 2 Urgench State University (UrSU), Urgench, Uzbekistan Correspondence: Ikram Abdullaev, Chairman Khorezm Academy of Mamun, 220900, Khiva, Uzbekistan. Tel: 998-362-375-8051. E-mail: [email protected] Received: June 12, 2019 Accepted: July 5, 2019 Online Published: July 9, 2019 doi:10.5539/ijb.v11n4p1 URL: https://doi.org/10.5539/ijb.v11n4p1 Abstract A variety of morphological and functional features of the digestive system of termites is associated with their nutritional adaptation. Wood is mostly the food of termites’ adult larvae, workers and young nymphs. The salivary and intestinal enzymes play an important part in this process. The physiology and biochemistry of the digestion system of termites from the genus Anacanthotermes is still not fully studied. In the present research, we studied the activity of some carbohydrases in termites’ salivary glands. Our data show that the activity of exocellulase in adult termites is 1.5 times more that in young individuals and 3 times more active than in nymphs, while the exocellulase in soldiers remains inactive. Moreover, the activity of celluloses in the intestine of A. turkestanicus is still not fully studied. We observed that exocellulase is involved in the digestion of food polymers in the castes of termites-workers, nymphs and soldiers. -
KS3 Science Textbook Sample
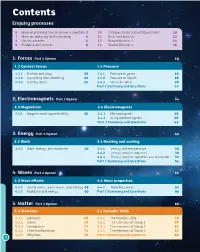
Contents Enquiry processes 6 More on planning how to answer a question 2 10 Critique claims and justify opinions 10 7 More on analysing and evaluating 4 11 Risks and benefits 12 8 Communication 6 12 Review theories 1 14 9 Evidence and sources 8 13 Review theories 2 16 1: Forces Part 2 Opener 18 1.3 Contact forces 1.4 Pressure 1.3.1 Friction and drag 20 1.4.1 Pressure in gases 26 1.3.2 Squashing and stretching 22 1.4.2 Pressure in liquids 28 1.3.3 Turning forces 24 1.4.3 Stress on solids 30 Part 2 Summary and Questions 32 2: Electromagnets Part 2 Opener 34 2.3 Magnetism 2.4 Electromagnets 2.3.1 Magnets and magnetic fields 36 2.4.1 Electromagnets 38 2.4.2 Using electromagnets 40 Part 2 Summary and Questions 42 3: Energy Part 2 Opener 44 3.3 Work 3.4 Heating and cooling 3.3.1 Work, energy, and machines 46 3.4.1 Energy and temperature 48 3.4.2 Energy transfer: particles 50 3.4.3 Energy transfer: radiation and insulation 52 Part 2 Summary and Questions 54 4: Waves Part 2 Opener 56 4.3 Wave effects 4.4 Wave properties 4.3.1 Sound waves, water waves, and energy 58 4.4.1 Modelling waves 62 4.3.2 Radiation and energy 60 Part 2 Summary and Questions 64 5: Matter Part 2 Opener 66 5.3 Elements 5.4 Periodic Table 5.3.1 Elements 68 5.4.1 The Periodic Table 78 5.3.2 Atoms 70 5.4.2 The elements of Group 1 80 5.3.3 Compounds 72 5.4.3 The elements of Group 7 82 5.3.4 Chemical formulae 74 5.4.4 The elements of Group 0 84 5.3.5 Polymers 76 Part 2 Summary and Questions 86 ii 6: Reactions Part 2 Opener 88 6.3 Types of reaction 6.4 Chemical energy 6.3.1 Atoms -

Carbohydrase and Phytase Supplementation in Diets for Semi-Heavy Laying Hens
Acta Scientiarum http://www.uem.br/acta ISSN printed: 1806-2636 ISSN on-line: 1807-8672 Doi: 10.4025/actascianimsci.v36i3.21952 Carbohydrase and phytase supplementation in diets for semi-heavy laying hens Adriano Geraldo1*, Karina Rodrigues Aurora Gomes1, Édison José Fassani2, Antonio Gilberto Bertechini2, Sérgio Domingos Simão1 and Filipe Soares Nogueira1 1Departamento de Ciências Agrárias, Instituto Federal de Educação Ciência e Tecnologia de Minas Gerais, Fazenda Varginha, km 05, Rodovia Bambuí-Medeiros, Cx. Postal 05, 38900-000, Bambuí, Minas Gerais, Brazil. 2Departamento de Zootecnia, Universidade Federal de Lavras, Lavras, Minas Gerais, Brazil. *Author for correspondence. E-mail: [email protected] ABSTRACT. This study was conducted in order to evaluate the association of phytase with an enzymatic complex comprised of carbohydrases (α-galactosidase, galactomannan, xylanase and β-glucanase) in nutrition reduction diets for semi-heavy laying hens and its effect on egg performance and egg quality. Four hundred Isa Brown laying hens with 42 to 57 weeks of age were distributed in an entirely random experiment with five treatments and 8 repetitions, during five production periods of 21 days. Variables studied: egg production, feed intake, mean egg weight, feed conversion, Haugh unit, percentage of yolk, egg white and albumen, yolk color, eggshell thickness and specific gravity. There was a significant interaction (p < 0.05) between treatments and experimental periods for feed intake. There were no significant effects (p > 0.05) of treatment on production, egg weight or internal and external egg quality. Treatment effects on feed conversion showed better values for hens fed with the control diet. The levels of nutrient reduction used in the diets with or without enzyme supplementation did not provide good results with regard to feed conversion and feed intake. -

Malt Carbohydrase
MALT CARBOHYDRASE Prepared at the 15th JECFA (1971), published in NMRS 50B (1972) and in FNP 52 (1992). An ADI 'not limited' was established at the 15th JECFA (1971) SYNONYMS Malt SOURCES Malt is the product of controlled germination of barley Active principles 1. alpha-Amylase (glycogenase, diastase) 2. ß-Amylase (glycogenase, diastase) Systematic names and 1. 1,4-alpha-D-Glucan glucanohydrolase (EC 3.2.1.1) numbers 2. 1,4-alpha-D-Glucan maltohydrolase (EC 3.2.1.2) Reactions catalyzed 1. Hydrolysis of 1,4-alpha-glucosidic linkages in polysaccharides, (starch, glycogen) yielding dextrins and oligo- and monosaccharides. 2. Hydrolysis of 1,4-alpha-glucosidic linkages in polysaccharides, (starch, glycogen) yielding successively maltose units from the non-reducing ends of the chains. FUNCTIONAL USES Enzyme preparation Used in brewing, baking, manufacture of alcoholic beverages and manufacture of syrups. GENERAL Must conform to the General Specifications for Enzyme Preparations used SPECIFICATIONS in Food Processing (see Volume Introduction) CHARACTERISTICS IDENTIFICATION alpha-Amylase activity The sample shows alpha-amylase activity (Vol. 4) alpha- and ß-amylase The sample shows Diastatic power activity See description under TESTS TESTS IDENTIFICATION TESTS Diastatic power Determination of a diastatic power of malt (combined activity of alpha- and ß-amylase) according to Amer. Soc. Brewing Chem., 6th ed., 162 (1958). Reagents For Digestion of Starch Solution - Acetate buffer solution: Dissolve 68 g of sodium acetate (CH3COONa · 3H2O), reagent grade, in 500 ml of one N acetic acid and make the solution up to one litre with distilled water. - Sodium hydroxide solution: 0.5 N - Special starch: Starch manufactured specifically for diastatic power determination is available from Merck & Co., Rahway, New Jersey. -

An Alpha-Amylase Assay, a Reagent Composition and a Reagent System Therefor
Patentamt JEuropâischesEuropean Patent Office © Publication number : 0 005 867 Office européen des brevets B2 © NEW EUROPEAN PATENT SPECIFICATION © Date of publication of the new patent spécification : © int. ci* : G 01 N 33/50, C 1 2 Q 1/40 09.07.86 © Application number : 79101791.6 © Date of filing : 06.06.79 © An alpha-amylase assay, a reagent composition and a reagent System therefor. (30)© Priority : 06.06.78 US 912986 © Proprietor : FLOW GENERAL, INC. 7655 Old Springhouse Road © Date of publication of application : Mclean Virginia 22102 (US) 12.12.79 Bulletin 79/25 © Inventor : Nix, Paul Thomas Publication of the grant of the patent : 62 Forest Drive 27.10.82 Bulletin 82/43 Jackson N.J. 08627 (US) Inventor : Goldfarb, Rebecca Dickstein Mention of the opposition décision : 224 Alexander Avenue 09.07.86 Bulletin 86/28 Howell N.J. 07731 (US) Inventor : Morgenstern, Stanley Walter 3805 Route 33 © Designated contracting states : N.J. 07753 DE FR Neptune (US) GB IT Inventor : Stong, Linda Jean 6 Blue Spruce Court R.D. 1 Références cited : Hightstown N.J. 08520 (US) DE-A- 2 705 859 Inventor : Sulick, Lorraine Eilen DE-A- 2 729 636 14 Winding Way FR-A- 2 402 872 Short Hills N.J. 07078 (US) US-A- 3 879 263 Inventor : Trivedi, Ramesh Chandulal Clinical Chemistry, vol. 24, no. 6, (1978), page 1000 110 W.Main Street Freehold N.J. 07728 (US) © Représentative : Vossius Vossius Tauchner Heune- Rauh csi mann Siebertstrasse 4 P.O. Box 86 07 67 D-8000 Mûnchen 86 (DE) <0 00 m o o Jouve, 18, rue St-Denis, 75001 Paris, France This invention relates to reagents and methods for determining alpha-amylase concentration in a body fluid, such as plasma, serum, urine and saliva.