Further Interpretation of Wodehouseia Spinata Stanley from the Late Maastrichtian of the Far East (China) M
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Alpine Garden Club of B.C. Bulletin
Alpine Garden Club of B.C. Vol.48, No. 4 Bulletin FALL 2005 Web Address: http://www.agc-bc.ca President Doug Smith 604-596-8489 1st V.P. Moya Drummond 604-738-6570 2nd V.P. Philip MacDougall 604-580-3219 Past President Ian Plenderleith 604-733-1604 Secretary Ian Gillam 4040 W.38th Ave, 604-266-6318 Vancouver, BC. V6N 2Y9 Treasurer Amanda Offers 604-885-7532 Editor Sue Evanetz 604-885-3356 3731 Beach Ave, RR 22, Roberts Creek, BC, V0N 2W2 Program Philip MacDougall 604-580-3219 14776-90th Ave, Surrey, BC, V3R 1A4 Pot Show Ellen Smith 604-596-8489 Membership Moya Drummond 604-738-6570 3307 West 6th Avenue, Vancouver, BC, V6R 1T2 Seed Exch. Ian & Phyllis Plenderleith, 604-733-1604 2237 McBain Ave, Vancouver, BC, V6L 3B2 Library Pam Frost 604-266-9897 6269 Elm St. BC, V6N 1B2, BC, V6N 1B2 Spring Show: Ian Gillam – as above Plant Sales: Mark Demers 604-254-5479 2222 Napier St, Vancouver, BC, V5N 2P2 Committee Members Mark Demers, Len Gardiner, Sara Jones, Joe Keller, Jason Nehring, Stuart Scholefield Honorary Life Members Rosemary Burnham, Margaret Charlton, Grace Conboy, Francisca Darts, Frank Dorsey, Pam Frost, Daphne Guernsey, Bodil Leamy, Jim MacPhail, Vera Peck, Geoff Williams, Bob Woodward Meetings are held the second Wednesday of each month except July & August, in the Floral Hall, VanDusen Botanical Garden. Doors and Library open at 7:00pm and Meetings start at 7:30pm sharp with the educational talk. Don’t forget to bring a prize for the raffle which goes a long way to paying for the hall rental. -

Fair Use of This PDF File of Herbaceous
Fair Use of this PDF file of Herbaceous Perennials Production: A Guide from Propagation to Marketing, NRAES-93 By Leonard P. Perry Published by NRAES, July 1998 This PDF file is for viewing only. If a paper copy is needed, we encourage you to purchase a copy as described below. Be aware that practices, recommendations, and economic data may have changed since this book was published. Text can be copied. The book, authors, and NRAES should be acknowledged. Here is a sample acknowledgement: ----From Herbaceous Perennials Production: A Guide from Propagation to Marketing, NRAES- 93, by Leonard P. Perry, and published by NRAES (1998).---- No use of the PDF should diminish the marketability of the printed version. This PDF should not be used to make copies of the book for sale or distribution. If you have questions about fair use of this PDF, contact NRAES. Purchasing the Book You can purchase printed copies on NRAES’ secure web site, www.nraes.org, or by calling (607) 255-7654. Quantity discounts are available. NRAES PO Box 4557 Ithaca, NY 14852-4557 Phone: (607) 255-7654 Fax: (607) 254-8770 Email: [email protected] Web: www.nraes.org More information on NRAES is included at the end of this PDF. Acknowledgments This publication is an update and expansion of the 1987 Cornell Guidelines on Perennial Production. Informa- tion in chapter 3 was adapted from a presentation given in March 1996 by John Bartok, professor emeritus of agricultural engineering at the University of Connecticut, at the Connecticut Perennials Shortcourse, and from articles in the Connecticut Greenhouse Newsletter, a publication put out by the Department of Plant Science at the University of Connecticut. -

Circumscription and Phylogeny of Apiaceae Subfamily Saniculoideae Based on Chloroplast DNA Sequences
ARTICLE IN PRESS Molecular Phylogenetics and Evolution xxx (2007) xxx–xxx www.elsevier.com/locate/ympev Circumscription and phylogeny of Apiaceae subfamily Saniculoideae based on chloroplast DNA sequences Carolina I. Calviño a,b,¤, Stephen R. Downie a a Department of Plant Biology, University of Illinois at Urbana-Champaign, Urbana, IL 61801-3707, USA b Instituto de Botánica Darwinion, Buenos Aires, Argentina Received 14 July 2006; revised 3 January 2007; accepted 4 January 2007 Abstract An estimate of phylogenetic relationships within Apiaceae subfamily Saniculoideae was inferred using data from the chloroplast DNA trnQ-trnK 5Ј-exon region to clarify the circumscription of the subfamily and to assess the monophyly of its constituent genera. Ninety- one accessions representing 14 genera and 82 species of Apiaceae were examined, including the genera Steganotaenia, Polemanniopsis, and Lichtensteinia which have been traditionally treated in subfamily Apioideae but determined in recent studies to be more closely related to or included within subfamily Saniculoideae. The trnQ-trnK 5Ј-exon region includes two intergenic spacers heretofore underutilized in molecular systematic studies and the rps16 intron. Analyses of these loci permitted an assessment of the relative utility of these noncoding regions (including the use of indel characters) for phylogenetic study at diVerent hierarchical levels. The use of indels in phylogenetic anal- yses of both combined and partitioned data sets improves resolution of relationships, increases bootstrap support values, and decreases levels of overall homoplasy. Intergeneric relationships derived from maximum parsimony, Bayesian, and maximum likelihood analyses, as well as from maximum parsimony analysis of indel data alone, are fully resolved and consistent with one another and generally very well supported. -

Seed Plant Phylogeny: Demise of the Anthophyte Hypothesis? Michael J
bb10c06.qxd 02/29/2000 04:18 Page R106 R106 Dispatch Seed plant phylogeny: Demise of the anthophyte hypothesis? Michael J. Donoghue* and James A. Doyle† Recent molecular phylogenetic studies indicate, The first suggestions that Gnetales are related to surprisingly, that Gnetales are related to conifers, or angiosperms were based on several obvious morphological even derived from them, and that no other extant seed similarities — vessels in the wood, net-veined leaves in plants are closely related to angiosperms. Are these Gnetum, and reproductive organs made up of simple, results believable? Is this a clash between molecules unisexual, flower-like structures, which some considered and morphology? evolutionary precursors of the flowers of wind-pollinated Amentiferae, but others viewed as being reduced from Addresses: *Harvard University Herbaria, 22 Divinity Avenue, Cambridge, Massachusetts 02138, USA. †Section of Evolution and more complex flowers in the common ancestor of Ecology, University of California, Davis, California 95616, USA. angiosperms, Gnetales and Mesozoic Bennettitales [1]. E-mail: [email protected] These ideas went into eclipse with evidence that simple [email protected] flowers really are a derived, rather than primitive, feature Current Biology 2000, 10:R106–R109 of the Amentiferae, and that vessels arose independently in angiosperms and Gnetales. Vessels in angiosperms 0960-9822/00/$ – see front matter seem derived from tracheids with scalariform pits, whereas © 2000 Elsevier Science Ltd. All rights reserved. in Gnetales they resemble tracheids with circular bor- dered pits, as in conifers. Gnetales are also like conifers in These are exciting times for those interested in plant lacking scalariform pitting in the primary xylem, and in evolution. -

On the Evolution of Angiosperms in the Himalayan Region: a Summary
The Palaeobotanist 57(2008) : 453-457 0031-0174/2008 $2.00 On the evolution of Angiosperms in the Himalayan region: A summary J.S. GULERIA Birbal Sahni Institute of Palaeobotany, 53 University Road, Lucknow 226007, India. [email protected] (Received 10 June, 2007; revised version accepted 15 September, 2008) ABSTRACT Guleria JS 2008. On the evolution of Angiosperms in the Himalayan region: A summary. The Palaeobotanist 57(3): 453-457. The paper summarises the evolution of angiosperms in different zones of Himalaya. The Himalayan Cenozoic flora has been divided age-wise as Palaeogene and Neogene flora. The Himalayan Palaeogene flora is largely a continuation of tropical peninsular flora of India. The early Miocene flora of Lesser Himalaya is also moist tropical. However, temperate plants started appearing during Miocene in the Higher Himalaya and their occurrence in Plio-Pleistocene flora of Kashmir reflect uplift of the Himalaya. The sub-Himalayan flora indicates existence of warm humid conditions in this belt which became drier by the end of Pliocene. The northern floral elements appeared to have invaded India all along the Himalayan belt. Since its birth the Himalaya has played a significant role in the immigration of plants from the adjoining regions, i.e. east, west and north, thereby enriching the Indian flora. The development of the Cenozoic flora of the Himalayan region is an expression of changing patterns of geography, topography and climate. Key-words—Cenozoic, Angiosperms, Himalayan flora, Migration, Climate (India). fgeky;h -

Ontogenetic Studies on the Determination of the Apical Meristem In
Ontogenetic studies on the determination of the apical meristem in racemose inflorescences D i s s e r t a t i o n Zur Erlangung des Grades Doktor der Naturwissenschaften Am Fachbereich Biologie Der Johannes Gutenberg-Universität Mainz Kester Bull-Hereñu geb. am 19.07.1979 in Santiago Mainz, 2010 CONTENTS SUMMARY OF THE THESIS............................................................................................ 1 ZUSAMMENFASSUNG.................................................................................................. 2 1 GENERAL INTRODUCTION......................................................................................... 3 1.1 Historical treatment of the terminal flower production in inflorescences....... 3 1.2 Structural understanding of the TF................................................................... 4 1.3 Parallel evolution of the character states referring the TF............................... 5 1.4 Matter of the thesis.......................................................................................... 6 2 DEVELOPMENTAL CONDITIONS FOR TERMINAL FLOWER PRODUCTION IN APIOID UMBELLETS...................................................................................................... 7 2.1 Introduction...................................................................................................... 7 2.2 Materials and Methods..................................................................................... 9 2.2.1 Plant material.................................................................................... -

Angiotensin Converting Enzyme Inhibitory Activity of Melinjo (Gnetum Gnemon L.) Seed Extracts and Molecular Docking of Its Stilbene Constituents
Online - 2455-3891 Vol 10, Issue 3, 2017 Print - 0974-2441 Research Article ANGIOTENSIN CONVERTING ENZYME INHIBITORY ACTIVITY OF MELINJO (GNETUM GNEMON L.) SEED EXTRACTS AND MOLECULAR DOCKING OF ITS STILBENE CONSTITUENTS ABDUL MUN’IM1*, MUHAMMAD ASHAR MUNADHIL1, NURAINI PUSPITASARI1, AZMINAH2, ARRY YANUAR2 1Department of Pharmacognosy-Phytochemistry, Faculty of Pharmacy, Universitas Indonesia, Depok 16424, Indonesia. 2Department of Biomedical Computation, Faculty of Pharmacy, Universitas Indonesia, Depok 16424, Indonesia. Email: [email protected] Received: 10 November 2016, Revised and Accepted: 30 November 2016 ABSTRACT Objectives: To evaluate the angiotensin converting enzyme (ACE) inhibitory activity of melinjo (Gnetum gnemon) seed extract and to study molecular docking of stilbene contained in melinjo seeds. Methods: Melinjo seed powders were extracted with n-hexane, dichloromethane, ethyl acetate, methanol, and water successively. The extracts were evaluated ACE inhibitory activities using ACE kit-Wist and the phenolic content using Folin–Ciocalteu method. The extract demonstrated the highest ACE inhibitory activity was subjected to liquid chromatography-mass spectrometry (LC-MS) to know its stilbene constituent. The stilbene constituents in melinjo seed were performed molecular docking using AutoDock Vina, and ligand-receptor Interactions were processed using Ligand Scout. Results: The ethyl acetate extract demonstrated the highest ACE inhibition activity with inhibitory concentration 50% value of 9.77 × 10 −8 μg/mL inhibitor),and the highest as in silicototal phenolicmolecular content docking (575.9 studies mg demonstrated gallic acid equivalent/g). that they fit Ultra-performance into the lisinopril receptors.LC-MS analysis of ethyl acetate extract has detected the existency of resveratrol, gnetin C, ε-viniferin, and gnemonoside A/B. -

Shri R. L. T College of Science, Akola 2019-2020 Department of Botany
Shri R. L. T College of Science, Akola 2019-2020 Department of Botany B. Sc-1 Semester-II Paleobotany, Gymnosperm, Morphology and Utilization of Plants Solve the Following MCQ’s Test Max.Marks:30 Time:60Mins 1) The study of past geological plant life is done in branch called as ….. a)Botany b)Paleobotany c)History d)Anthropology 2)The father of Indian paleobotany is…. a)Homi Bhabha b) C.V. Raman c)Maheshwari d)Birbal Sahani 3) The earth is supposed to be originated before…. a) 4.6 billion years ago b)3.4 billion years ago c) 4.6 million years ago d)none of these 4)The oldest era in which life is originated was on the earth. a)proterozoic era b)Archeozoic era c)Paleozoic era d)Mesozoic era 5)The following era is called as “age of ferns” a)proterozoic era b)Archeozoic era c)paleozoic era d)Mesozoic era 6) The following era is called as era of Modern Life…. a)Coenozoic era b)Archeozoic era c)Paleozoic era d)Mesozoic era 7) Sphenopteris hoeninghausii is the….. a)stem b)root c)leaves d)ovule 8) Root of L.oldhamia is called as…. a) Sphenopteris hoeninghausii b)Kaloxylon hookeri c)Lagenostoma lomaxii d)Rachiopteris aspera 9) Cycadeoidea is also known as…. a)Lyginopteris b)Bennettites c) Pinus d)Gnetum 10) The manoxylic wood refers to........... a)single ring of xylem b)hard and compressed c)loose and soft d)many rings of xylem. 11) Endosperm in gymnosperm is............. a) haploid b)diploid c)triploid d)tetraploid 12) The ovules in gymnosperms are... -
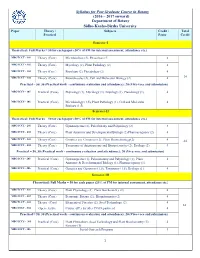
Syllabus for Post Graduate Course in Botany (2016 – 2017 Onward)
Syllabus for Post Graduate Course in Botany (2016 – 2017 onward) Department of Botany Sidho-Kanho-Birsha University Paper Theory / Subjects Credit / Total Practical Paper Credit Semester-I Theoretical: Full Marks = 50 for each paper (20% of FM for internal assessment, attendance etc.) MBOTCCT - 101 Theory (Core) Microbiology (2), Phycology (2) 4 MBOTCCT - 102 Theory (Core) Mycology (2), Plant Pathology (2) 4 MBOTCCT - 103 Theory (Core) Bryology (2), Pteridology (2) 4 MBOTCCT - 104 Theory (Core) Biomolecules (2), Cell and Molecular Biology (2) 4 24 Practical = 50, 30 (Practical work - continuous evaluation and attendance); 20 (Viva-voce and submission) MBOTCCS - 105 Practical (Core) Phycology (1), Mycology (1), Bryology (1), Pteridology (1). 4 MBOTCCS - 106 Practical (Core) Microbiology (1.5), Plant Pathology (1), Cell and Molecular 4 Biology (1.5). Semester-II Theoretical: Full Marks = 50 for each paper (20% of FM for internal assessment, attendance etc.) MBOTCCT - 201 Theory (Core) Gymnosperms (2), Paleobotany and Palynology (2) 4 MBOTCCT - 202 Theory (Core) Plant Anatomy and Developmental Biology (2) Pharmacognosy (2) 4 MBOTCCT - 203 Theory (Core) Genetics and Genomics (2), Plant Biotechnology(2) 4 24 MBOTCCT - 204 Theory (Core) Taxonomy of Angiosperms and Biosystematics (2), Ecology (2) 4 Practical = 50, 30 (Practical work - continuous evaluation and attendance); 20 (Viva-voce and submission) MBOTCCS - 205 Practical (Core) Gymnosperms (1), Palaeobotany and Palynology (1), Plant 4 Anatomy & Developmental Biology (1), Pharmacognosy (1). MBOTCCS - 206 Practical (Core) Genetics and Genomics (1.5), Taxonomy (1.5), Ecology (1). 4 Semester-III Theoretical: Full Marks = 50 for each paper (20% of FM for internal assessment, attendance etc.) MBOTCCT - 301 Theory (Core) Plant Physiology (2), Plant Biochemistry (2) 4 MBOTCCT - 302 Theory (Core) Economic Botany (2), Bioinformatics (2) 4 MBOTCCT - 303 Theory (Core) Elements of Forestry (2), Seed Technology (2). -

PG and Research Deparment of Botany Pteridophytes, Gymnosperms and Paleobotany
PG and Research Deparment of Botany Pteridophytes, Gymnosperms and Paleobotany Part-A 1. The vascular tissue is confined to the central region of the stem forming: (a) Bundles (b) stele (c) Cortex (d) Pericycle 2. The leaves which bear the sporangia are called: (a) sporophylIs (b) Bract (c) Cone (d) Strobilus 3. One or two peripheral layers persist for the nourishment of the developing spores. These nourishing cells form: (a) Elators (b) Sopores (c) Jacket (d) tapetum. 4. Match gametophyte with one of the followings: (a) Prothallus (b) Thallus (c) Cone (d) Strobilus 5. Selaginella belongs to division (a) Lycopsida (b) Pteropsida (c) Psilopsida (d) Sphenopsida 6. Hone tails are: (a) Lycopsida (b) Pteropsida (c ) Psilopsida (d) Sphenopsida 7. Marsilea belongs: (a) Lycopsida (b) Pteropsida (c) Psilopsida (d) Sphenopsida 8. Which of the followings is fern? (a) Psilopsida (b) Pteropsida (c) Lycopsida (d) Sphenopsida 9. Which of the followings is most primitive division? (a) Lycopsida (b) Pteropsida (c) Psilopsida (d) Sphenopsida 10. Club mosses are: (a) Lycopsida (b) Pteropsida (c) Psilopsida (d) Sphenopsida 11. The protostele in which xylem core is Smooth and rounded is: (a) Haplostele (b) Actinostlele (c) Plectostele (d) Siphonostele 12. The protostele in which xylem core is star like is called: (a) Haplostele (b) Actinostlele (c) Plectostele (d) Siphonostele 13. The siphonostele in–which two cylinders of vascular tissue are present in the stele is: (a) Haplostele (b) Actinostlele (c) Plectostele (d) Polycyclic 14. In Xylem in which protoxylem is lying in the middle of Metaxylem is: (a) Exarch (b) Mesarch (c) Endarch (d) Diarch 15. -

Collector's & Bulb Lists
175 Arbutus Rd. Salt Spring Island, B.C. V8K 1A3 Ph/ Fax(250) 537-5788 Specializing in Native, Rare and Unusual Plants at www.thimblefarms.com Hours of Operation: February Through December: Open 9-4:30 daily; July through August we will be open but how much will depend on staffing- please phone before coming to the nursery. Welcome to our 2009 catalogue. With the nursery and, indeed, most of the country, locked in a winter wonderland of ice and snow it seems like a good time to plan and dream about when we can escape into the oases of our own gardens to relax, enjoy the simpler things and escape the turmoil that surrounds us. With that in mind, we have endeavored to bring you a bevy of new plant treasures from around the world so you can explore the possibility of adding something new, rare or exotic to your garden. Perhaps you are thinking of enhancing an existing garden, or developing something with a theme, for example an Asian garden, or more specifically a Kashmir bed or Himalayan border. Maybe you’ve always dreamed about filling a bed with Cypripediums and other exotic, but hardy, orchids, expanding a woodland bed with a fernery or creating a native garden. Regardless of your vision, we have something new for you to try. Throughout the catalogue you will find new additions to almost all groups: Ferns, Hardy Orchids, Cypripediums, Hostas, Native plants, Sarracenias and more. We have also worked hard to bring back popular treasures we once offered like the double Anemonellas, Himalayan Maidenhair Fern and Arisaemas, just to name a few. -

The Ephedra, the Gnetum and the Welwitschia. Genus
MODULE I UNIT 4 THE PHYLUM GNETOPHYTA This phylum consists of three genera; the Ephedra, the Gnetum and the Welwitschia. Genus: Ephedra There are about 100 known species of gnetophytes. They are unique among the gymnosperms in having vessels in the xylem. More than half of the gnetophytes are species of joint firs in the genus Ephedra. These shrubby plants inhabit drier regions of southwestern North America. Their tiny leaves are produced in twos and threes at a node and turn brown soon after they appear. The stems and branches, which are often whorled, are slightly ribbed; they are photosynthetic when they are young (Fig. 14). The leaves are little more than scales; therefore, most photosynthesis is conducted by the green stem. Before pollination, the ovules of Ephedra produce a small tubular extension resembling the neck of a miniature bottle extending into the air. Sticky fluid oozes out of this extension, which constitutes the micropyle, and airborne pollen catches in the fluid. Male and female strobili may be produced on the same plant or on different ones, depending on the species. Figure 14: Joint fir (Ephedra) 1 Economic Importance of Ephedra Joint fir is the source of the drug ephedrine, an alkaloid that constricts swollen blood vessels and also a mild stimulant. An overdose can cause death. It is also used as a tea in Chinese herbal medicine. Genus: Gnetum The members of Gnetum occur in the tropics of Africa, South America, and South Asia. Most are vine-like, with broad leaves similar to those of flowering plants (Fig.15).