FTA Drug & Alcohol Regulation Updates
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The National Drugs List
^ ^ ^ ^ ^[ ^ The National Drugs List Of Syrian Arab Republic Sexth Edition 2006 ! " # "$ % &'() " # * +$, -. / & 0 /+12 3 4" 5 "$ . "$ 67"5,) 0 " /! !2 4? @ % 88 9 3: " # "$ ;+<=2 – G# H H2 I) – 6( – 65 : A B C "5 : , D )* . J!* HK"3 H"$ T ) 4 B K<) +$ LMA N O 3 4P<B &Q / RS ) H< C4VH /430 / 1988 V W* < C A GQ ") 4V / 1000 / C4VH /820 / 2001 V XX K<# C ,V /500 / 1992 V "!X V /946 / 2004 V Z < C V /914 / 2003 V ) < ] +$, [2 / ,) @# @ S%Q2 J"= [ &<\ @ +$ LMA 1 O \ . S X '( ^ & M_ `AB @ &' 3 4" + @ V= 4 )\ " : N " # "$ 6 ) G" 3Q + a C G /<"B d3: C K7 e , fM 4 Q b"$ " < $\ c"7: 5) G . HHH3Q J # Hg ' V"h 6< G* H5 !" # $%" & $' ,* ( )* + 2 ا اوا ادو +% 5 j 2 i1 6 B J' 6<X " 6"[ i2 "$ "< * i3 10 6 i4 11 6! ^ i5 13 6<X "!# * i6 15 7 G!, 6 - k 24"$d dl ?K V *4V h 63[46 ' i8 19 Adl 20 "( 2 i9 20 G Q) 6 i10 20 a 6 m[, 6 i11 21 ?K V $n i12 21 "% * i13 23 b+ 6 i14 23 oe C * i15 24 !, 2 6\ i16 25 C V pq * i17 26 ( S 6) 1, ++ &"r i19 3 +% 27 G 6 ""% i19 28 ^ Ks 2 i20 31 % Ks 2 i21 32 s * i22 35 " " * i23 37 "$ * i24 38 6" i25 39 V t h Gu* v!* 2 i26 39 ( 2 i27 40 B w< Ks 2 i28 40 d C &"r i29 42 "' 6 i30 42 " * i31 42 ":< * i32 5 ./ 0" -33 4 : ANAESTHETICS $ 1 2 -1 :GENERAL ANAESTHETICS AND OXYGEN 4 $1 2 2- ATRACURIUM BESYLATE DROPERIDOL ETHER FENTANYL HALOTHANE ISOFLURANE KETAMINE HCL NITROUS OXIDE OXYGEN PROPOFOL REMIFENTANIL SEVOFLURANE SUFENTANIL THIOPENTAL :LOCAL ANAESTHETICS !67$1 2 -5 AMYLEINE HCL=AMYLOCAINE ARTICAINE BENZOCAINE BUPIVACAINE CINCHOCAINE LIDOCAINE MEPIVACAINE OXETHAZAINE PRAMOXINE PRILOCAINE PREOPERATIVE MEDICATION & SEDATION FOR 9*: ;< " 2 -8 : : SHORT -TERM PROCEDURES ATROPINE DIAZEPAM INJ. -

Skeletal Muscle Relaxants Review 12/17/2008
Skeletal Muscle Relaxants Review 12/17/2008 Copyright © 2007 - 2008 by Provider Synergies, L.L.C. All rights reserved. Printed in the United States of America. All rights reserved. No part of this publication may be reproduced or transmitted in any form or by any means, electronic or mechanical, including photocopying, recording, digital scanning, or via any information storage and retrieval system without the express written consent of Provider Synergies, L.L.C. All requests for permission should be mailed to: Attention: Copyright Administrator Intellectual Property Department Provider Synergies, L.L.C. 5181 Natorp Blvd., Suite 205 Mason, Ohio 45040 The materials contained herein represent the opinions of the collective authors and editors and should not be construed to be the official representation of any professional organization or group, any state Pharmacy and Therapeutics committee, any state Medicaid Agency, or any other clinical committee. This material is not intended to be relied upon as medical advice for specific medical cases and nothing contained herein should be relied upon by any patient, medical professional or layperson seeking information about a specific course of treatment for a specific medical condition. All readers of this material are responsible for independently obtaining medical advice and guidance from their own physician and/or other medical professional in regard to the best course of treatment for their specific medical condition. This publication, inclusive of all forms contained herein, is intended to -

Drug Class Review on Skeletal Muscle Relaxants
Drug Class Review on Skeletal Muscle Relaxants Final Report Update 2 May 2005 Original Report Date: April 2003 Update 1 Report Date: January 2004 A literature scan of this topic is done periodically The purpose of this report is to make available information regarding the comparative effectiveness and safety profiles of different drugs within pharmaceutical classes. Reports are not usage guidelines, nor should they be read as an endorsement of, or recommendation for, any particular drug, use or approach. Oregon Health & Science University does not recommend or endorse any guideline or recommendation developed by users of these reports. Roger Chou, MD Kim Peterson, MS Oregon Evidence-based Practice Center Oregon Health & Science University Mark Helfand, MD, MPH, Director Copyright © 2005 by Oregon Health & Science University Portland, Oregon 97201. All rights reserved. Note: A scan of the medical literature relating to the topic is done periodically (see http://www.ohsu.edu/ohsuedu/research/policycenter/DERP/about/methods.cfm for scanning process description). Upon review of the last scan, the Drug Effectiveness Review Project governance group elected not to proceed with another full update of this report. Some portions of the report may not be up to date. Prior versions of this report can be accessed at the DERP website. Final Report Update 2 Drug Effectiveness Review Project TABLE OF CONTENTS Introduction........................................................................................................................4 Scope and -

Acetaminophen and Methocarbamol
PATIENT & CAREGIVER EDUCATION Acetaminophen and Methocarbamol This information from Lexicomp® explains what you need to know about this medication, including what it’s used for, how to take it, its side effects, and when to call your healthcare provider. Brand Names: Canada Muscle & Back Pain Relief [OTC]; Robaxacet [OTC] Warning Liver problems have happened with the use of acetaminophen. Sometimes, this has led to a liver transplant or death. Most of the time, liver problems happened in people taking more than 4,000 mg (milligrams) of acetaminophen in a day. People were also often taking more than 1 drug that had acetaminophen in it. If you have questions, talk with your doctor. What is this drug used for? It is used to ease pain. It is used to relax muscles. What do I need to tell my doctor BEFORE I take this drug? If you are allergic to this drug; any part of this drug; or any other drugs, foods, or substances. Tell your doctor about the allergy and what signs you had. If you have liver disease. Acetaminophen and Methocarbamol 1/7 If you have taken certain drugs used for low mood (depression) like isocarboxazid, phenelzine, or tranylcypromine or drugs used for Parkinson’s disease like selegiline in the last 14 days. Taking this drug within 14 days of those drugs can cause very bad high blood pressure. If you are taking any of these drugs: Linezolid or methylene blue. If you are taking another drug that has the same drug in it. This is not a list of all drugs or health problems that interact with this drug. -

Medicaid Drug Use Criteria
Medicaid Drug Use Criteria Skeletal Muscle Relaxants ● Developed October 2008. ● Revised June 2020; May 2018; May 2016; September 2014; December 2012; March 2011. Information on indications for use or diagnosis is assumed to be unavailable. All criteria may be applied retrospectively; prospective application is indicated with an asterisk [*]. The information contained is for the convenience of the public. The Texas Health and Human Services Commission is not responsible for any errors in transmission or any errors or omissions in the document. Medications listed in the tables and non-FDA approved indications included in these retrospective criteria are not indicative of Vendor Drug Program formulary coverage. Prepared by: ● Drug Information Service, UT Health San Antonio ● The College of Pharmacy, the University of Texas at Austin 1 Dosage 1.1 Adults The skeletal muscle relaxants (SMRs), carisoprodol, chlorzoxazone, cyclobenzaprine, methocarbamol, metaxalone, and orphenadrine, are FDA- approved for short-term use to manage discomfort associated with acute, painful musculoskeletal conditions such as strains, sprains, and other muscle injuries. These agents should be used as an adjunct to non-pharmacologic treatments, 1 including rest and physical therapy. The SMRs, baclofen, dantrolene, and tizanidine are FDA-approved for managing spasticity due to upper motor neuron disorders (e.g., spinal cord injury, cerebral palsy, multiple sclerosis, stroke). Maximum recommended dosages for SMRs are summarized in Table 1. Dosages exceeding these recommendations -
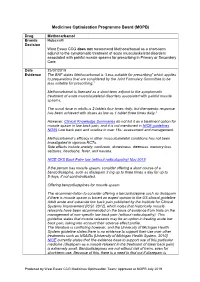
Medicines Optimisation Programme Board (MOPB)
Medicines Optimisation Programme Board (MOPB) Drug Methocarbamol Brands Robaxin® Decision West Essex CCG does not recommend Methocarbamol as a short-term adjunct to the symptomatic treatment of acute musculoskeletal disorders associated with painful muscle spasms for prescribing in Primary or Secondary Care. Date 25/07/2019 Evidence The BNF states Methocarbamol is “Less suitable for prescribing” which applies to preparations that are considered by the Joint Formulary Committee to be less suitable for prescribing.1 Methocarbamol is licensed as a short-term adjunct to the symptomatic treatment of acute musculoskeletal disorders associated with painful muscle spasms. The usual dose in adults is 2 tablets four times daily, but therapeutic response has been achieved with doses as low as 1 tablet three times daily.2 However, Clinical Knowledge Summaries do not list it as a treatment option for muscle spasm in low back pain, and it is not mentioned in NICE guidelines NG59 Low back pain and sciatica in over 16s: assessment and management. Methocarbamol’s efficacy in other musculoskeletal conditions has not been investigated in rigorous RCTs. Side effects include anxiety, confusion, drowsiness, dizziness, memory loss, seizures, headache, fever, and nausea. NICE CKS Back Pain- low (without radiculopathy) Nov 2018 If the person has muscle spasm, consider offering a short course of a benzodiazepine, such as diazepam 2 mg up to three times a day for up to 5 days, if not contraindicated. Offering benzodiazepines for muscle spasm The recommendation to consider offering a benzodiazepine such as diazepam if there is muscle spasm is based on expert opinion in the US clinical guideline Adult acute and subacute low back pain published by the Institute for Clinical Systems Improvement [ICSI, 2012], which notes that historically muscle relaxants have been recommended on the basis of evidence from trials on the management of non-specific low back pain (without radiculopathy). -

Skeletal Muscle Relaxants Review 05/31/2010
Skeletal Muscle Relaxants Review 05/31/2010 Copyright © 2007 – 2010 by Provider Synergies, L.L.C. All rights reserved. Printed in the United States of America. All rights reserved. No part of this publication may be reproduced or transmitted in any form or by any means, electronic or mechanical, including photocopying, recording, digital scanning, or via any information storage and retrieval system without the express written consent of Provider Synergies, L.L.C. All requests for permission should be mailed to: Attention: Copyright Administrator Intellectual Property Department Provider Synergies, L.L.C 10101 Alliance Road, Suite 201 Cincinnati, Ohio 45242 The materials contained herein represent the opinions of the collective authors and editors and should not be construed to be the official representation of any professional organization or group, any state Pharmacy and Therapeutics committee, any state Medicaid Agency, or any other clinical committee. This material is not intended to be relied upon as medical advice for specific medical cases and nothing contained herein should be relied upon by any patient, medical professional or layperson seeking information about a specific course of treatment for a specific medical condition. All readers of this material are responsible for independently obtaining medical advice and guidance from their own physician and/or other medical professional in regard to the best course of treatment for their specific medical condition. This publication, inclusive of all forms contained herein, is intended -

Analgesia for Non-Intubated Patients
Practice Management Guidelines for Analgesia in the Non-Intubated Patient Division of Trauma and Surgical Critical Care I. Rationale: Multimodal analgesia (MMA) regimens are defined as the use of a variety of medications to target different physiologic mechanisms of action in either the central and/or peripheral nervous system to effect pain relief. [1] MMA has been shown through randomized trials to result in overall better pain relief with less opioid consumption compared to single modality pain regimens. [2,3] MMA includes, but is not limited to, usage of acetaminophen, non-steroidal anti-inflammatory drugs, muscle relaxants, narcotics, and gabapentinoids. Locoregional and neuraxial blockade can also serve as important adjuncts in MMA regimens. II. Algorithm Patient with Acute Pain and NO Enteral Access Patient with preexisting opiate use and/or risk factors for difficult analgesic control? • Chronic opiate use • Active IV drug Use YES Consider Acute Pain • Suboxone or methadone use Service consultation • Chronic pain/fibromyalgia • Pain refractory to measures outlined within this PMG • Meets APS consult criteria per Rib fracture & APS Guidelines PMG NPO Multimodal Regimen: -PRN hydromorphone if patient unable to manage PCA -Scheduled acetaminophen PR -Scheduled ketorolac IV (if no contraindications) - Scheduled or PRN methocarbamol IV (if having muscle spasms) -Lidocaine patch if complaining of localized pain Considerations for PCA use NPO or not tolerating PO meds? NO Patient alert? Able to manage/understand PCA use? YES Hydromorphone -

(IJAR) ISSN 2332-2780 Incidence and Severity of Suxamethonium
OPEN ACCESS https://scidoc.org/IJBRP.php International Journal of Anesthesiology & Research (IJAR) ISSN 2332-2780 Incidence And Severity Of Suxamethonium Induced Fasciculation And Post-Operative Myalgia, And Their Association With Different Iv Induction Agents Among Adult Patients Underwent Elective Sur- gery At Jimma Medical Center, A Prospective Cohort Study Research Article Mebratu Tila1, Zemenu Muluken2, Negashu Dadi2, Mesfin Zewude3, Elias Habtu1, Getahun Dendir1, WonduReta Demissie4* 1 Wolaita Sodo University, College of medicine and Health Sciences, School of Anesthesia, WolaitaSodo, Ethiopia. 2 Jimma University, Institute of Health, Faculty of Medical Science, School of Anesthesia, Jimma, Ethiopia. 3 Jimma University, Institute of Technology, Jimma, Ethiopia. 4 Jimma University, Institute of Health, Faculty of Medical Science, Department of Biomedical sciences, Jimma, Ethiopia. Abstract Background: Suxamethonium induced fasciculation (SIF) and post-operative myalgia (POM) is one the side effects occurred following administration of suxamethonium. Objective: The present study aimed to assess the incidence and severity of SIF and POM, and their association with different IV induction agents among adult patients underwent elective surgery at Jimma medical center (JMC). Methods: Prospective cohort study design was employed among a sampled 140 patients whoinduced by four IV induction agents (propofol, thiopentone, ketamine and ketofol) where in each group 35 patients were equally distributed. SIF and POM were assessed by separated structured grading and scoring toolsintraoperatively and post-operatively (respectively) after expos- ing patients toalready mentioned four induction agents. Data was entered into Epidata version 4.3.1 and finally exported to SPSS version 20 for further analysis. Cross tabulation/chi square and binary logistic regression were applied to determine their association. -

An Anticholinergic Burden Score for German Prescribers: Score Development Esther Katharina Kiesel1* , Yvonne Marina Hopf1 and Michael Drey2
Kiesel et al. BMC Geriatrics (2018) 18:239 https://doi.org/10.1186/s12877-018-0929-6 RESEARCH ARTICLE Open Access An anticholinergic burden score for German prescribers: score development Esther Katharina Kiesel1* , Yvonne Marina Hopf1 and Michael Drey2 Abstract Background: Anticholinergic drugs put elderly patients at a higher risk for falls, cognitive decline, and delirium as well as peripheral adverse reactions like dry mouth or constipation. Prescribers are often unaware of the drug- based anticholinergic burden (ACB) of their patients. This study aimed to develop an anticholinergic burden score for drugs licensed in Germany to be used by clinicians at prescribing level. Methods: A systematic literature search in pubmed assessed previously published ACB tools. Quantitative grading scores were extracted, reduced to drugs available in Germany, and reevaluated by expert discussion. Drugs were scored as having no, weak, moderate, or strong anticholinergic effects. Further drugs were identified in clinical routine and included as well. Results: The literature search identified 692 different drugs, with 548 drugs available in Germany. After exclusion of drugs due to no systemic effect or scoring of drug combinations (n = 67) and evaluation of 26 additional identified drugs in clinical routine, 504 drugs were scored. Of those, 356 drugs were categorised as having no, 104 drugs were scored as weak, 18 as moderate and 29 as having strong anticholinergic effects. Conclusions: The newly created ACB score for drugs authorized in Germany can be used in daily clinical practice to reduce potentially inappropriate medications for elderly patients. Further clinical studies investigating its effect on reducing anticholinergic side effects are necessary for validation. -
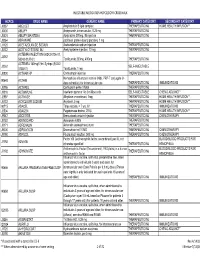
Injectable Medication Hcpcs/Dofr Crosswalk
INJECTABLE MEDICATION HCPCS/DOFR CROSSWALK HCPCS DRUG NAME GENERIC NAME PRIMARY CATEGORY SECONDARY CATEGORY J0287 ABELCET Amphotericin B lipid complex THERAPEUTIC INJ HOME HEALTH/INFUSION** J0400 ABILIFY Aripiprazole, intramuscular, 0.25 mg THERAPEUTIC INJ J0401 ABILIFY MAINTENA Apriprazole 300mg, IM injection THERAPEUTIC INJ J9264 ABRAXANE paclitaxel protein-bound particles, 1 mg J1120 ACETAZOLAMIDE SODIUM Acetazolamide sodium injection THERAPEUTIC INJ J0132 ACETYLCYSTEINE INJ Acetylcysteine injection, 10 mg THERAPEUTIC INJ ACTEMRA INJECTION (50242-0136-01, J3262 50242-0137-01) Tocilizumab 200mg, 400mg THERAPEUTIC INJ ACTEMRA 162mg/0.9ml Syringe (50242- J3262 SELF-INJECTABLE 0138-01) Tocilizumab, 1 mg J0800 ACTHAR HP Corticotropin injection THERAPEUTIC INJ Hemophilus influenza b vaccine (Hib), PRP-T conjugate (4- 90648 ACTHIB dose schedule), for intramuscular use THERAPEUTIC INJ IMMUNIZATIONS J0795 ACTHREL Corticorelin ovine triflutal THERAPEUTIC INJ J9216 ACTIMMUNE Interferon gamma 1-b 3 miillion units SELF-INJECTABLE CHEMO ADJUNCT* J2997 ACTIVASE Alteplase recombinant, 1mg THERAPEUTIC INJ HOME HEALTH/INFUSION** J0133 ACYCLOVIR SODIUM Acyclovir, 5 mg THERAPEUTIC INJ HOME HEALTH/INFUSION** 90715 ADACEL Tdap vaccine, > 7 yrs, IM THERAPEUTIC INJ IMMUNIZATIONS J2504 ADAGEN Pegademase bovine, 25 IU THERAPEUTIC INJ HOME HEALTH/INFUSION** J9042 ADCETRIS Brentuximab vedotin Injection THERAPEUTIC INJ CHEMOTHERAPY J0153 ADENOCARD Adenosine 6 MG THERAPEUTIC INJ J0171 ADRENALIN Adrenalin (epinephrine) inject THERAPEUTIC INJ J9000 ADRIAMYCIN Doxorubicin -

Review of Existing Classification Efforts
Project No. TREN-05-FP6TR-S07.61320-518404-DRUID DRUID Driving under the Influence of Drugs, Alcohol and Medicines Integrated Project 1.6. Sustainable Development, Global Change and Ecosystem 1.6.2: Sustainable Surface Transport 6th Framework Programme Deliverable 4.1.1 Review of existing classification efforts Due date of deliverable: (15.01.2008) Actual submission date: (07.02.2008) Start date of project: 15.10.2006 Duration: 48 months Organisation name of lead contractor for this deliverable: UGent Revision 1.0 Project co-funded by the European Commission within the Sixth Framework Programme (2002-2006) Dissemination Level PU Public X PP Restricted to other programme participants (including the Commission Services) RE Restricted to a group specified by the consortium (including the Commission Services) CO Confidential, only for members of the consortium (including the Commission Services) Task 4.1 : Review of existing classification efforts Authors: Kristof Pil, Elke Raes, Thomas Van den Neste, An-Sofie Goessaert, Jolien Veramme, Alain Verstraete (Ghent University, Belgium) Partners: - F. Javier Alvarez (work package leader), M. Trinidad Gómez-Talegón, Inmaculada Fierro (University of Valladolid, Spain) - Monica Colas, Juan Carlos Gonzalez-Luque (DGT, Spain) - Han de Gier, Sylvia Hummel, Sholeh Mobaser (University of Groningen, the Netherlands) - Martina Albrecht, Michael Heiβing (Bundesanstalt für Straßenwesen, Germany) - Michel Mallaret, Charles Mercier-Guyon (University of Grenoble, Centre Regional de Pharmacovigilance, France) - Vassilis Papakostopoulos, Villy Portouli, Andriani Mousadakou (Centre for Research and Technology Hellas, Greece) DRUID 6th Framework Programme Deliverable D.4.1.1. Revision 1.0 Review of Existing Classification Efforts Page 2 of 127 Introduction DRUID work package 4 focusses on the classification and labeling of medicinal drugs according to their influence on driving performance.