High-Performance Resist Materials for Arf Excimer Laser and Electron Beam Lithography
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Advanced Excimer Laser Technologies Enable Green Semiconductor Manufacturing
PROCEEDINGS OF SPIE SPIEDigitalLibrary.org/conference-proceedings-of-spie Advanced excimer laser technologies enable green semiconductor manufacturing Hitomi Fukuda, Youngsun Yoo, Yuji Minegishi, Naoto Hisanaga, Tatsuo Enami Downloaded From: https://www.spiedigitallibrary.org/conference-proceedings-of-spie on 10/25/2017 Terms of Use: https://spiedigitallibrary.spie.org/ss/TermsOfUse.aspx Advanced Excimer Laser Technologies Enable Green Semiconductor Manufacturing Hitomi Fukuda*, Youngsun Yoo, Yuji Minegishi, Naoto Hisanaga and Tatsuo Enami Gigaphoton Inc., 400 Yokokura-Shinden, Oyama-shi, Tochigi, JAPAN 323-8558 ABSTRACT "Green" has fast become an important and pervasive topic throughout many industries worldwide. Many companies, especially in the manufacturing industries, have taken steps to integrate green initiatives into their high-level corporate strategies. Governments have also been active in implementing various initiatives designed to increase corporate responsibility and accountability towards environmental issues. In the semiconductor manufacturing industry, there are growing concerns over future environmental impact as enormous fabs expand and new generation of equipments become larger and more powerful. To address these concerns, Gigaphoton has implemented various green initiatives for many years under the EcoPhoton™ program. The objective of this program is to drive innovations in technology and services that enable manufacturers to significantly reduce both the financial and environmental “green cost” of laser operations in high-volume manufacturing environment (HVM) – primarily focusing on electricity, gas and heat management costs. One example of such innovation is Gigaphoton’s Injection-Lock system, which reduces electricity and gas utilization costs of the laser by up to 50%. Furthermore, to support the industry’s transition from 300mm to the next generation 450mm wafers, technologies are being developed to create lasers that offer double the output power from 60W to 120W, but reducing electricity and gas consumption by another 50%. -

I. Excimer Fluorescence, II. Heavy-Atom Spin-Orbital Coupling Effect and Iii. the Electronic Spectra of Ferrocene." (1965)
Louisiana State University LSU Digital Commons LSU Historical Dissertations and Theses Graduate School 1965 Studies in Molecular Spectroscopy; I. Excimer Fluorescence, II. Heavy-Atom Spin-Orbital Coupling Effect and IIi. The lecE tronic Spectra of Ferrocene. Fred Jewel Smith Louisiana State University and Agricultural & Mechanical College Follow this and additional works at: https://digitalcommons.lsu.edu/gradschool_disstheses Recommended Citation Smith, Fred Jewel, "Studies in Molecular Spectroscopy; I. Excimer Fluorescence, II. Heavy-Atom Spin-Orbital Coupling Effect and IIi. The Electronic Spectra of Ferrocene." (1965). LSU Historical Dissertations and Theses. 1092. https://digitalcommons.lsu.edu/gradschool_disstheses/1092 This Dissertation is brought to you for free and open access by the Graduate School at LSU Digital Commons. It has been accepted for inclusion in LSU Historical Dissertations and Theses by an authorized administrator of LSU Digital Commons. For more information, please contact [email protected]. This dissertation has bssn microfilmed exactly as received 6 6 -7 4 8 SMITH, Fred Jewel, 1939- STUDIES IN MOLECULAR SPECTROSCOPY} I. EXCIMER FLUORESCENCE, II. HEAVY- ATOM SPIN-ORBITAL COUPLING EFFECT AND HI. THE ELECTRONIC SPECTRA OF FERROCENE. Louisiana State University, Ph.D., 1965 Chemistry, physical University Microfilms, Inc., Ann Arbor, Michigan STUDIES IN MOLECULAR SPECTROSCOPY; I. EXCIMER FLUORESCENCE, II. HEAVY-ATOM SPIN-ORBITAL COUPLING EFFECT AND III . THE ELECTRONIC SPECTRA OF FERROCENE A Dissertation Submitted to the Graduate Faculty of the Louisiana State University and Agricultural and Mechanical College in partial fulfillment of the requirements for the degree of Doctor of Philosophy in The Department of Chemistry by Fred Jewel Smith B.A., University of Southern Mississippi, 1960 August, 1965 ACKNOWLEDGMENT The author wishes to express his sincere appreciation to Dr. -

6 5 • Vacuum Ultraviolet Ar Excimer Emission Initiated by High Intensity Laser Produced Electrons
JP0050803 JAERI-Cont" 2000-006 6 5 • Vacuum Ultraviolet Ar Excimer Emission Initiated by High Intensity Laser Produced Electrons Shoichi Kubodera and Wataru Sasaki Department of Electrical and Electronic Engineering and Photon Science Center, Miyazaki University Gakuen Kibanadai Nishi 1-1, Miyazaki, 889-2192 Japan We have observed Ar2* emission using a tabletop femtosecond high intensity laser as an excitation source. High intensity laser produced electrons via an optical field induced ionization (OFI) process initiated the Ar2* production kinetics, which made themselves analogous to those produced in an electron beam produced plasma. A fast conductive cooling of the OFI plasma was found to be appropriate to initiate the excimer formation kinetics more efficiently. Keywords: Vacuum ultraviolet, Excimer molecule, Short pulse laser, Conductive cooling There have been considerable demands for the development of compact short wavelength lasers in the vacuum ultraviolet (VUV) spectral region. Such compact short wavelength lasers would be applicable to various scientific and industrial fields, such as photochemistry, biological science, and new types of materials processing. Currently available practical compact VUV lasers are the ArF excimer laser at 193 tun and the F2 laser at 157 run, both of which are excited by a compact discharge device. Recently more attention is paid to short wavelength lasers in the VUV in the future optical lithography industry. Rare gas excimers have long been one of the very few laser media in the VUV spectral region [1]. The emission wavelength of Ar2* is 126 run which is long enough to use transmission optical elements such as MgF2 and LiF. The Kr2* laser has an even longer emission wavelength centered at 147 mn which relaxes the conditions for optics and would become a competitor to the F2 laser at 157 run. -

Laser Induced Breakdown Spectroscopy for Elemental Analysis in Environmental, Cultural Heritage and Space Applications: a Review of Methods and Results
Sensors 2010, 10, 7434-7468; doi:10.3390/s100807434 OPEN ACCESS sensors ISSN 1424-8220 www.mdpi.com/journal/sensors Review Laser Induced Breakdown Spectroscopy for Elemental Analysis in Environmental, Cultural Heritage and Space Applications: A Review of Methods and Results Rosalba Gaudiuso 1,*, Marcella Dell’Aglio 2, Olga De Pascale 2, Giorgio S. Senesi 2 and Alessandro De Giacomo 1,2 1 Department of Chemistry, University of Bari, via Orabona 4, 79126, Bari, Italy; E-Mail: [email protected] 2 IMIP-CNR sec. Bari, via Amendola 122/D, 70126, Bari, Italy; E-Mails: [email protected] (M.D.A.); [email protected] (O.P.); [email protected] (G.S.S.) * Author to whom correspondence should be addressed; E-Mail: [email protected]; Tel.: +39-080-5929522; +39-080-5929513; Fax: +39-080-5929520. Received: 7 April 2010; in revised form: 24 May 2010 / Accepted: 22 June 2010 / Published: 9 August 2010 Abstract: Analytical applications of Laser Induced Breakdown Spectroscopy (LIBS), namely optical emission spectroscopy of laser-induced plasmas, have been constantly growing thanks to its intrinsic conceptual simplicity and versatility. Qualitative and quantitative analysis can be performed by LIBS both by drawing calibration lines and by using calibration-free methods and some of its features, so as fast multi-elemental response, micro-destructiveness, instrumentation portability, have rendered it particularly suitable for analytical applications in the field of environmental science, space exploration and cultural heritage. This review reports and discusses LIBS achievements in these areas and results obtained for soils and aqueous samples, meteorites and terrestrial samples simulating extraterrestrial planets, and cultural heritage samples, including buildings and objects of various kinds. -

Krf Laser Development A
L+ (j@ fw” 2675-V* / 03 Los Alamos National bboratoq is operated by the University of CaI~ornia for the United States Department of Energy under contract W-7405 -ENG-36 Lowwmm Acknowledgements Technical Review: David C. Cartwnght Joseph F. Figuena Thomas E. McDonald Publication Support: Pa[ncia A. Hinnebusch, C/tiefEdi[or Susrut H. Kinzer, Edi(ou Florence M. Butler, Reference Librarian Lucy P. Maestas, Production A4anagcmen( Mildred E. Valdez, Special Assistant Elizabeth Courtney, Word Processing, Graphics Susan L. Carlson, Graphic Design Support AnnMarie Dyson, llluwations Donna L. Duran, Illustration Support Wendy M. Burditt, Word ProcessinglComposition Joyce A. Martinez, Word ProccssinglComposition Jody Shepard, Assistant Dorothy Hatch, Word Processing Printing Coordination Guadalupe D. Archuleta This document was produced on a Macintosh IITM.LaserWriter Plusw, Varityper VT600Pm, and CanonTM Scanner using Microsoft Wordw v 3.01 and 4.0, Expressionist,w and Adobe Illustratorm. The work reported here was supported by the Office of Inertial Confinement Fusion, Department of Energy — Defense Programs. Front Cover Interior of A URORA target chamber looking directly through the center of the target chamber ton’ard the lens cone. The array of ~’bite circles containing rectangular red stripes in the center of this photograph is a portion of the lens pla(e, some three meters behind the target chamber n’hichfocuses the 48 laser beatns onto the target. The small yellon’ sphere in the center of the chamber is an optical reference target used for optical aiignntent of the laser beants. The ycllom tube entering the chumberfiom (he upper left is part of the x-ray diagnostics system. -

Excimer Laser Pure Mixtures
Excimer Laser Pure Mixtures Excimer laser cavities are charged with an optional mixture of a halogen donor, typically fluorine or hydrogen chloride, a rare gas buffer and an inert gas diluent, usually helium. Sometimes a small amount of hydrogen is added as a lifetime extension additive. The use of Matheson Tri-Gas pure gas components with halogen mixtures permits the optimal charging of the laser cavity to provide stable pulsed laser output, enhanced shot to shot amplitude stability, increased gas fill time and reduced system contamination. Argon Cylinder Contents Major Component Concentration Size* US Metric Valve Outlet In Helium Certified or Unanalyzed 1 - 25% 300 269 ft3 7.07 m3 CGA 580 200 200 ft3 5.26 m3 In Neon Certified or Unanalyzed 1 - 25% 300 265 ft3 6.97 m3 CGA 580 200 199 ft3 5.23 m3 Shipping Information: DOT Name: . .Rare Gases Mixtures Hazard Class: . 2.2 ID No.: . .UN 1979 DOT Label: . Nonflammable Gas *Other sizes available on request. Hydrogen Cylinder Contents Major Component Concentration Size* US Metric Valve Outlet In Helium Certified or Unanalyzed 0.02 - 0.1% 300 269 ft3 7.07 m3 CGA 350 200 200 ft3 5.26 m3 In Neon Certified or Unanalyzed 0.02 - 0.1% 300 265 ft3 6.97 m3 CGA 580 200 199 ft3 5.23 m3 Shipping Information: DOT Name: . Compressed Gases, N.O.S. Hazard Class: . 2.2 ID No.: . .UN 1956 DOT Label: . Nonflammable Gas *Other sizes available on request. Excimer Laser Pure Mixtures Hydrogen Chloride Cylinder Contents Major Component Concentration Size* US Metric Valve Outlet In Helium Certified or Unanalyzed 0.2 - 10% 300 269 ft3 7.07 m3 CGA 330 200 200 ft3 5.26 m3 In Neon Certified or Unanalyzed 0.2 - 10% 300 265 ft3 6.97 m3 CGA 330 200 199 ft3 5.23 m3 Shipping Information: (Must list two components in parentheses in association with the proper shipping name.) DOT Name: . -

Excimer and Free-Electron Lasers
I I £ S" 00 ?^ I RT/TIB/84/39 T. LETARDt, A. RENIERI THE LIGHT FOR THE FUTURE: EXCIMER AND FREE-ELECTRON LASERS COMITATO NAZIONALE PER LA RICERCA E PER LO SVILUPPO DELL'ENERGIA NUCLEARE E DELLE ENERGIE ALTERNATIVE COMITATO NAZIONALE PER LA RICERCA E PER LO SVILUPPO DELL'ENERGIA NUCLEARE E DELLE ENERGIE ALTERNATIVE THE LIGHT FOR THE FUTURE: =><* AND FREE-ELECTRON LASERS T. LETARDI, A. REHIERI Dipartimento TIB, Divisioue Fisica Applicata, C.R.E. Frascati g^CA;-. mYTIB/84/39 Testo pervenuto in dicembre 1984 This report has been prepared by: Servizio Studi e Docuaientazione • ENEA, Centro Ricerrhe Energia Fraacati, C.P. 65 - 00044 Frascati, Hone, Italy. Thia Office vili be glad to aend further copiea of thia report on requeat. The technical and scientific contents of theae reporta expreaa the opinioni o.' the authora but not necessarily "lose of ENEA Sjhmittrd to New Technologies in Neurosurgery ABSTRACT The status of art of two interesting kind of coherent radia tion sources (excimer and free-electron lasers) is described. The working principles of exciser lasers is analyzed together with the most relevant characteristics (active media, operating wave length, average and peak power, spectrum and time structure). As to the free-electron laser, whose development is at the very first stage, theoretical and experimental aspects are described and perspectives for the future are discussed. RIASSUNTO Viene descritto lo stato dell'arte di due tipi di sorgente laser che grande interesse hanno sollevato in questi ultimi anni, il laser ad eccimeri ed il laser ad ad elettroni liberi. Per il laser ad eccimeri, assieme a principi di funzionamento, vengono descritte le più importanti caratteristiche (mezzi attivi, lun ghezze d'onda di emissione, potenza media e di picco, caratteri stiche spettrali e temporali). -

The Argon Fluoride Laser As an Enabler for Low Cost Inertial Fusion Energy
The Argon Fluoride laser as an enabler for low cost inertial fusion energy ARPA-E FUSION Workshop August 13-14, 2019 San Francisco Airport Marriott Waterfront Presented by Stephen Obenschain Laser Plasma Branch Plasma Physics Division Why an ArF laser driver could enable lower cost modest size laser IFE power plants The superior laser target coupling with ArF’s deep UV light (193 nm) could enable the high target gains needed for the energy application at much lower laser energies than previously thought feasible. The combination of deep UV light and broad native bandwidth (>5 THz) suppresses laser-plasma instabilities that limit the laser intensity and ablation pressures of current 351 nm frequency-tripled glass lasers which are the traditional laser drivers for fusion. ArF is a potentially disruptive technology for laser fusion that shares several advantageous technologies with the krypton fluoride (KrF) laser technology (λ=248 nm) used on the Nike laser system located at the Naval Research Laboratory (NRL). The ArF laser would utilize similar electron-beam pumping to that used for large KrF amplifiers. It would also be able to use the beam smoothing technology demonstrated on Nike that enables very uniform illumination of directly driven targets and provides the capability to “zoom” the focal profile to follow an imploding target. The KrF technology was chosen for the Nike facility because of numerous advantages for achieving laser fusion. ArF laser light in turn would be superior to KrF. For the IFE application, kinetics simulations indicate that ArF would have as much as 1.6x higher intrinsic efficiency than KrF. -

Observation of Helium Excimer Clusters Generated Via Neutron-3He Absorption Reaction Department of Physics, Nagoya University Taku Matsushita
Observation of Helium Excimer Clusters Generated via Neutron-3He Absorption Reaction Department of Physics, Nagoya University Taku Matsushita 1. Introduction Turbulence is one of subjects which is scientifically and industrially important but whose detail remains unsolved by its complexity. Therefore, quantum turbulence, where vortices are quantized, has been expected to be a key to solve the physics of turbulence, in addition to scientific interests on the nature specific of quantized vortices. To study quantum turbulence existing in superfluid 4He, recently flow visualization using triplet metastable 4He molecules (excimers) He2* as a tracer was emerged to be a new method which provides us information on normal-component flow individually [Marakov et al., Phys. Rev. B (2015)]. However, complete observation of the velocity fields in turbulent superfluid 4He requires localized small clouds of He2* excimers, clusters, of which generation method has not been established so far. Thus, to realize such excimer clusters, we proposed the use of the neutron absorption reaction 3He(n,p)3H of 3He impurities contained in liquid 4He, where 1H and 3H resulted by the reaction have sufficient energy to excite 4He atoms for excimer generation. And, we started the proof-of-principle experiments using the neutron beam of J-PARC/MLF. As the first step, in previous experiment (Project No. 2016B0111), we found that the application of neutron beam increases the signal suggesting He2* excimer generation, but the reproducibility and stability were not sufficient for detailed analyses. Therefore, we improved the detection system as shown in the next section. Thus, this experiment was conducted to obtain the proof of excimer generation via neutron-3He absorption reaction by quantitative analyses of improved experimental data. -

Two-Wavelength Dye Laser Pumped by Excimer Laser
Optica Applicata, Vol. XXII, No. 2, 1992 Two-wavelength dye laser pumped by excimer laser L. Pokora, Z. P uzewicz, Z. U jda Institute of Quantum Electronics, ul. S. Kaliskiego 6, 01—489 Warszawa, Poland. A photodynamic cancer diagnosis and treatment system has been developed, using an excimer-dye laser as a light source. The diagnosis and treatment laser beams were alternately generated by one dye laser having two-cell dye cuvette. In initial laboratory testing being presently performed, the system is yielding encouraging results. 1. Introduction One of the more interesting applications of dye lasers is their use in medicine — in the photodynamic method of tumor treatment (PDT). In the initial phase of research, simple light bulbs, xenon lamps and others with high illumination intensity were used. Only the introduction of lasers, producing narrow beams of light which could be led through a flexible optical cable and introduced via the arm of an endoscope into internal organs had created the possibility of using the photodynamic method for treatment of almost all internal organs. The laser set appropriate for both the PDT method and hematoporphyrin type preparations is the one which generates radiation of about 405 nm wavelength for suitable diagnostic purposes and 630 nm for tumor therapy. The 405 nm radiation is optimal for the first absorption peak of porphyrines. As a result of light absorption at this wavelength the region becomes strongly fluorescent in the red light band. The laser light wavelength of 630 nm fits into the weaker peak of absorption, but assures greater penetration of the light into human tissue. -
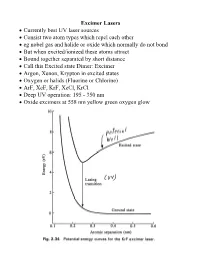
Excimer Lasers • Currently Best UV Laser Sources • Consist Two Atom
Excimer Lasers • Currently best UV laser sources • Consist two atom types which repel each other • eg nobel gas and halide or oxide which normally do not bond • But when excited/ionized these atoms attract • Bound together separated by short distance • Call this Excited state Dimer: Excimer • Argon, Xenon, Krypton in excited states • Oxygen or halids (Fluorine or Chlorine) • ArF, XeF, KrF, XeCl, KrCl • Deep UV operation: 195 - 350 nm • Oxide excimers at 558 nm yellow green oxygen glow Excimer Lasers • Mixture of gases below 5 atm • 90% - 99% buffer gas (He, Ne) for energy transfer • 1% - 9% dimer, 0.1-0.2% halide • Super-radiant lasers; 4% output mirror, reflective back • Quartz optics for XeCl • Fluorine would etch Quartz • Hence F lasers use magnesium fluoride or calcium fluoride • Cavity's under 1 m • To stabilize beam use injection locking with a Master Oscillator Power Amplifier (MOPA) Excimer dielectric mirrors Excimer Lasers • Excimer state short lived: pulses about 10 nsec • Repetition rate 100 - 300 Hz • Efficiency about 2% for KrF • Pulses highly unstable • 0.3 nm spectral width • Highly unstable modes: 2500 measured • Used for photoablation: wavelength so short destroys organics • KrF & ArF Main source for microelectronics exposure systems • Wavelength 249, 195 nm Vacuum UV: air absorbs Excimer Lasers • Excimer Lasers expensive to set up • Beam quality very poor • Emission area very large (eg 12x30 mm) • Bearly a laser • Need considerable support devices • Laser is heavy ~ 200-400 kg • Must have gas bottle cabinets (eg Xe & Cl) & ventilation system • Gas cabinets needed to catch leaks – deadly • Need a beam shaper to get good quality beam shapes wavefront so it can be focused ~ 10x • Costs high – laser $100K, but optics, gas cabinets another $100K Typical Excimer Lasers Chemical Laser • Chemical reaction to create laser action • Proposed by J.C. -

Ultraviolet 308-Nm Excimer Laser Ablation of Bone: an Acute and Chronic Study
Ultraviolet 308-nm excimer laser ablation of bone: an acute and chronic study J. Stuart Nelson, Arie Orenstein, Lih-Huei L. Liaw, Rhonda B. Zavar, Sanjay Gianchandani, and Michael W. Berns A comparative study was undertaken in rabbit tibiae to assess the healing of bone in response to osteotomies by a XeCl 308-nm excimer laser and a mechanical saw. The laser parameter producing the most efficient ablation rate for osteotomy was determined and shown to produce deep cuts with sharp edges. However, it was noted histologically that there was a significant delay in healing of the laser osteotomies compared with saw osteotomies. This delay was caused by thermal damage to bone adjacent to the laser cuts. It is suggested that the excimer laser at this wavelength ablates bone primarily through a photothermal as opposed to a photodissociative mechanism. I. Introduction remove precise amounts of tissue without generating Excimer lasers are used in photochemistry to pro- secondary heat effects. Long-term studies are need- voke photochemical reactions in research and produc- ed, but the prospects for early clinical use in corrective tion processes, including the enrichment of uranium.1' 2 refractive surgery seem good.5 They are also used in photolithography-for example, In dermatology, the extreme precision of the ex- to produce electronic circuits previously made by a cimer laser may be better than the traditional scalpel photographic process.3 Predictable amounts of sur- for removing bulky skin lesions. The 248-nm radia- face material can be removed, leaving etched marks tion has the ability to both produce a sharp skin inci- with microscopically sharp edges.