Relationships Between Cranial Base Synchondroses and Craniofacial Development: a Review Teddy Cendekiawan, Ricky W.K
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Joints Classification of Joints
Joints Classification of Joints . Functional classification (Focuses on amount of movement) . Synarthroses (immovable joints) . Amphiarthroses (slightly movable joints) . Diarthroses (freely movable joints) . Structural classification (Based on the material binding them and presence or absence of a joint cavity) . Fibrous mostly synarthroses . Cartilagenous mostly amphiarthroses . Synovial diarthroses Table of Joint Types Functional across Synarthroses Amphiarthroses Diarthroses (immovable joints) (some movement) (freely movable) Structural down Bony Fusion Synostosis (frontal=metopic suture; epiphyseal lines) Fibrous Suture (skull only) Syndesmoses Syndesmoses -fibrous tissue is -ligaments only -ligament longer continuous with between bones; here, (example: radioulnar periosteum short so some but not interosseous a lot of movement membrane) (example: tib-fib Gomphoses (teeth) ligament) -ligament is periodontal ligament Cartilagenous Synchondroses Sympheses (bone united by -hyaline cartilage -fibrocartilage cartilage only) (examples: (examples: between manubrium-C1, discs, pubic epiphyseal plates) symphesis Synovial Are all diarthrotic Fibrous joints . Bones connected by fibrous tissue: dense regular connective tissue . No joint cavity . Slightly immovable or not at all . Types . Sutures . Syndesmoses . Gomphoses Sutures . Only between bones of skull . Fibrous tissue continuous with periosteum . Ossify and fuse in middle age: now technically called “synostoses”= bony junctions Syndesmoses . In Greek: “ligament” . Bones connected by ligaments only . Amount of movement depends on length of the fibers: longer than in sutures Gomphoses . Is a “peg-in-socket” . Only example is tooth with its socket . Ligament is a short periodontal ligament Cartilagenous joints . Articulating bones united by cartilage . Lack a joint cavity . Not highly movable . Two types . Synchondroses (singular: synchondrosis) . Sympheses (singular: symphesis) Synchondroses . Literally: “junction of cartilage” . Hyaline cartilage unites the bones . Immovable (synarthroses) . -

Yagenich L.V., Kirillova I.I., Siritsa Ye.A. Latin and Main Principals Of
Yagenich L.V., Kirillova I.I., Siritsa Ye.A. Latin and main principals of anatomical, pharmaceutical and clinical terminology (Student's book) Simferopol, 2017 Contents No. Topics Page 1. UNIT I. Latin language history. Phonetics. Alphabet. Vowels and consonants classification. Diphthongs. Digraphs. Letter combinations. 4-13 Syllable shortness and longitude. Stress rules. 2. UNIT II. Grammatical noun categories, declension characteristics, noun 14-25 dictionary forms, determination of the noun stems, nominative and genitive cases and their significance in terms formation. I-st noun declension. 3. UNIT III. Adjectives and its grammatical categories. Classes of adjectives. Adjective entries in dictionaries. Adjectives of the I-st group. Gender 26-36 endings, stem-determining. 4. UNIT IV. Adjectives of the 2-nd group. Morphological characteristics of two- and multi-word anatomical terms. Syntax of two- and multi-word 37-49 anatomical terms. Nouns of the 2nd declension 5. UNIT V. General characteristic of the nouns of the 3rd declension. Parisyllabic and imparisyllabic nouns. Types of stems of the nouns of the 50-58 3rd declension and their peculiarities. 3rd declension nouns in combination with agreed and non-agreed attributes 6. UNIT VI. Peculiarities of 3rd declension nouns of masculine, feminine and neuter genders. Muscle names referring to their functions. Exceptions to the 59-71 gender rule of 3rd declension nouns for all three genders 7. UNIT VII. 1st, 2nd and 3rd declension nouns in combination with II class adjectives. Present Participle and its declension. Anatomical terms 72-81 consisting of nouns and participles 8. UNIT VIII. Nouns of the 4th and 5th declensions and their combination with 82-89 adjectives 9. -

1. Synarthrosis - Immovable
jAnatomy Lecture Notes Chapter 9 I. classification A. by function - 1. synarthrosis - immovable 2. amphiarthrosis - slightly movable 3. diarthrosis - freely movable B. by structure - material attaching bones together 1. fibrous -.dense c.t., no joint cavity a. suture - very thin, short fibers synostosis - ossification of fibrous c.t. in a suture joint b. syndesmosis - ligament (the longer the fibers the more movement is possible) c. gomphosis - periodontal ligament holds teeth in alveoli 2. cartilaginous - cartilage, no joint cavity a. synchondrosis - hyaline cartilage b. symphysis - fibrocartilage 3. synovial - joint capsule and ligaments II. structure of a synovial joint A. bone and articular cartilage (hyaline) • articular cartilage cushions bone ends by absorbing compression stress Strong/Fall 2008 page 1 jAnatomy Lecture Notes Chapter 9 B. articular capsule 1. fibrous capsule - dense irregular c.t.; holds bones together 2. synovial membrane - areolar c.t. with some simple squamous e.; makes synovial fluid C. joint cavity and synovial fluid 1. synovial fluid consists of: • fluid that is filtered from capillaries in the synovial membrane • glycoprotein molecules that are made by fibroblasts in the synovial membrane 2. fluid lubricates surface of bones inside joint capsule D. ligaments - made of dense fibrous c.t.; strengthen joint • capsular • extracapsular • intracapsular E. articular disc / meniscus - made of fibrocartilage; improves fit between articulating bones F. bursae - membrane sac enclosing synovial fluid found around some joints; cushion ligaments, muscles, tendons, skin, bones G. tendon sheath - elongated bursa that wraps around a tendon Strong/Fall 2008 page 2 jAnatomy Lecture Notes Chapter 9 III. movements at joints flexion extension abduction adduction circumduction rotation inversion eversion protraction retraction supination pronation elevation depression opposition dorsiflexion plantar flexion gliding Strong/Fall 2008 page 3 jAnatomy Lecture Notes Chapter 9 IV. -

3-Joints.Pdf
“In order to succeed, we must first believe that we can”. - Nikos Kazantzakis. objectives: - Define the term “Joint”. - Describe the classification of the 3 types of joints & give an example of each. - Describe the characteristics of synovial joints. - Describe the classification of synovial joints & give an example of each. - List factors maintaining stability of joints. - Recite “Hilton’s law” for nerve supply of joints. Joints: What is a joint? Classification of joints: It is the site where two or more bones meet According to the tissues that lie between the bones, Joints are classified into: together. What we mean by "two or more" in the Fibrous Cartilaginous Synovial definition is that at some articulations, - The articulating - The Two bone are - The bones are joined two or more bones might be joined together like the knee joint, where the surfaces are joined joined by cartilage by a fibrous capsule. femur, tibia and patella articulate by fibrous tissue together by a synovial joint. - The articular surfaces are covered by a thin layer of hyaline cartilage 1-Fibrous Joints: - The articulating surfaces are joined by : fibrous tissue - Movement: No or very mild movement - Examples: -inferior tibiofibular -Skull Sutures- joints (syndesmosis)- -Gomphosis- Temporary (ossify later) - Minimal movement dental alveolar joints. - Permanent joints. Between the teeth and there socket. 2-Cartilaginous Joints: - The two bones are joined by : Cartilage. The main difference between the primary and the secondary cartilaginous joints is that the primary is joined by a plate of hyaline cartilage, meanwhile the secondary is joined by a - TYPES: small amount of fibrous tissue forming a plate of fibrocartilage. -
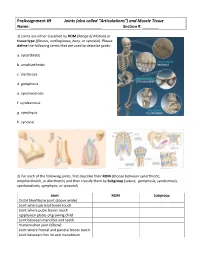
Preassignment #9 Joints (Also Called “Articulations”) and Muscle Tissue Name: Section #: __
PreAssignment #9 Joints (also called “Articulations”) and Muscle Tissue Name: _______________________________ Section #: _______ 1) Joints are either classified by ROM (Range of Motion) or tissue type (fibrous, cartilaginous, bony, or synovial). Please define the following terms that are used to describe joints: a. synarthrotic b. amphiarthrotic c. diarthrotic d. gomphosis e. synchondrosis f. syndesmosis g. symphysis h. synovial 2) For each of the following joints, first describe their ROM (choose between synarthrotic, amphiarthrotic, or diarthrotic) and then classify them by Subgroup (suture, gomphosis, syndesmosis, synchondrosis, symphysis, or synovial). Joint ROM Subgroup Distal tibiofibular joint (above ankle) Joint where parietal bones touch Joint where pubic bones touch Epiphyseal plates of growing child Joint between mandible and teeth Humeroulnar joint (elbow) Joint where frontal and parietal bones touch Joint between first rib and manubrium 3) Please label the parts of the generic synovial joint below. Use the terms: synovial membrane, ligament, periosteum, articular cartilage, synovial fluid, fibrous capsule, and joint capsule. Now, to see if you really understand joints, can you add labels for the diaphyseal cavity and epiphyseal line? 4) Synovial joints (or diarthroses) are usually classified as plane, hinge, saddle, ball-and-socket, or pivot joints. Note: I don’t list condyloid as a subgroup since it is really just a modified form of the hinges! For each of the following joints, please identify the classification to which they belong: a. humeroradial b. scapulohumeral c. interphalangeal d. atloaxis e. carpometacarpal #1 f. intercarpals g. proximal radioulnar h. sacroiliac 5) Movements at a joint are referred to as actions. For each of the following statements, tell me the proper action that is being used: a. -

Terminologia Anatomica and Its Practical Usage: Pitfalls and How to Avoid Them
CORE Metadata, citation and similar papers at core.ac.uk Provided by Via Medica Journals ONLINE FIRST This is a provisional PDF only. Copyedited and fully formatted version will be made available soon. ISSN: 0015-5659 e-ISSN: 1644-3284 Terminologia Anatomica and its practical usage: pitfalls and how to avoid them Authors: Piotr Paweł Chmielewski, Zygmunt Antoni Domagała DOI: 10.5603/FM.a2019.0086 Article type: REVIEW ARTICLES Submitted: 2019-06-29 Accepted: 2019-07-10 Published online: 2019-07-29 This article has been peer reviewed and published immediately upon acceptance. It is an open access article, which means that it can be downloaded, printed, and distributed freely, provided the work is properly cited. Articles in "Folia Morphologica" are listed in PubMed. Powered by TCPDF (www.tcpdf.org) Terminologia Anatomica and its practical usage: pitfalls and how to avoid them Running title: New Terminologia Anatomica and its practical usage Piotr Paweł Chmielewski, Zygmunt Antoni Domagała Division of Anatomy, Department of Human Morphology and Embryology, Faculty of Medicine, Wroclaw Medical University Address for correspondence: Dr. Piotr Paweł Chmielewski, PhD, Division of Anatomy, Department of Human Morphology and Embryology, Faculty of Medicine, Wroclaw Medical University, 6a Chałubińskiego Street, 50-368 Wrocław, Poland, e-mail: [email protected] ABSTRACT In 2016, the Federative International Programme for Anatomical Terminology (FIPAT) tentatively approved the updated and extended version of anatomical terminology that replaced the previous version of Terminologia Anatomica (1998). This modern version has already appeared in new editions of leading anatomical atlases and textbooks, including Netter’s Atlas of Human Anatomy, even though it was originally available only as a draft and the final version is different. -

4 Anat 35 Articulations
Human Anatomy Unit 1 ARTICULATIONS In Anatomy Today Classification of Joints • Criteria – How bones are joined together – Degree of mobility • Minimum components – 2 articulating bones – Intervening tissue • Fibrous CT or cartilage • Categories – Synarthroses – no movement – Amphiarthrosis – slight movement – Diarthrosis – freely movable Synarthrosis • Immovable articulation • Types – Sutures – Schindylesis – Gomphosis – Synchondrosis Synarthrosis Sutures • Found only in skull • Immovable articulation • Flat bones joined by thin layer of fibrous CT • Types – Serrate – Squamous (lap) – Plane Synarthrosis Sutures • Serrate • Serrated edges of bone interlock • Two portions of frontal bones • Squamous (lap) • Overlapping beveled margins forms smooth line • Temporal and parietal bones • Plane • Joint formed by straight, nonoverlapping edges • Palatine process of maxillae Synarthrosis Schindylesis • Immovable articulation • Thin plate of bone into cleft or fissure in a separation of the laminae in another bone • Articulation of sphenoid bone and perpendicular plate of ethmoid bone with vomer Synarthrosis Gomphosis • Immovable articulation • Conical process into a socket • Articulation of teeth with alveoli of maxillary bone • Periodontal ligament = fibrous CT Synarthrosis Synchondrosis • Cartilagenous joints – Ribs joined to sternum by hyaline cartilage • Synostoses – When joint ossifies – Epiphyseal plate becomes epiphyseal line Amphiarthrosis • Slightly moveable articulation • Articulating bones connected in one of two ways: – By broad flattened -

26 April 2010 TE Prepublication Page 1 Nomina Generalia General Terms
26 April 2010 TE PrePublication Page 1 Nomina generalia General terms E1.0.0.0.0.0.1 Modus reproductionis Reproductive mode E1.0.0.0.0.0.2 Reproductio sexualis Sexual reproduction E1.0.0.0.0.0.3 Viviparitas Viviparity E1.0.0.0.0.0.4 Heterogamia Heterogamy E1.0.0.0.0.0.5 Endogamia Endogamy E1.0.0.0.0.0.6 Sequentia reproductionis Reproductive sequence E1.0.0.0.0.0.7 Ovulatio Ovulation E1.0.0.0.0.0.8 Erectio Erection E1.0.0.0.0.0.9 Coitus Coitus; Sexual intercourse E1.0.0.0.0.0.10 Ejaculatio1 Ejaculation E1.0.0.0.0.0.11 Emissio Emission E1.0.0.0.0.0.12 Ejaculatio vera Ejaculation proper E1.0.0.0.0.0.13 Semen Semen; Ejaculate E1.0.0.0.0.0.14 Inseminatio Insemination E1.0.0.0.0.0.15 Fertilisatio Fertilization E1.0.0.0.0.0.16 Fecundatio Fecundation; Impregnation E1.0.0.0.0.0.17 Superfecundatio Superfecundation E1.0.0.0.0.0.18 Superimpregnatio Superimpregnation E1.0.0.0.0.0.19 Superfetatio Superfetation E1.0.0.0.0.0.20 Ontogenesis Ontogeny E1.0.0.0.0.0.21 Ontogenesis praenatalis Prenatal ontogeny E1.0.0.0.0.0.22 Tempus praenatale; Tempus gestationis Prenatal period; Gestation period E1.0.0.0.0.0.23 Vita praenatalis Prenatal life E1.0.0.0.0.0.24 Vita intrauterina Intra-uterine life E1.0.0.0.0.0.25 Embryogenesis2 Embryogenesis; Embryogeny E1.0.0.0.0.0.26 Fetogenesis3 Fetogenesis E1.0.0.0.0.0.27 Tempus natale Birth period E1.0.0.0.0.0.28 Ontogenesis postnatalis Postnatal ontogeny E1.0.0.0.0.0.29 Vita postnatalis Postnatal life E1.0.1.0.0.0.1 Mensurae embryonicae et fetales4 Embryonic and fetal measurements E1.0.1.0.0.0.2 Aetas a fecundatione5 Fertilization -

FIPAT-TA2-Part-2.Pdf
TERMINOLOGIA ANATOMICA Second Edition (2.06) International Anatomical Terminology FIPAT The Federative International Programme for Anatomical Terminology A programme of the International Federation of Associations of Anatomists (IFAA) TA2, PART II Contents: Systemata musculoskeletalia Musculoskeletal systems Caput II: Ossa Chapter 2: Bones Caput III: Juncturae Chapter 3: Joints Caput IV: Systema musculare Chapter 4: Muscular system Bibliographic Reference Citation: FIPAT. Terminologia Anatomica. 2nd ed. FIPAT.library.dal.ca. Federative International Programme for Anatomical Terminology, 2019 Published pending approval by the General Assembly at the next Congress of IFAA (2019) Creative Commons License: The publication of Terminologia Anatomica is under a Creative Commons Attribution-NoDerivatives 4.0 International (CC BY-ND 4.0) license The individual terms in this terminology are within the public domain. Statements about terms being part of this international standard terminology should use the above bibliographic reference to cite this terminology. The unaltered PDF files of this terminology may be freely copied and distributed by users. IFAA member societies are authorized to publish translations of this terminology. Authors of other works that might be considered derivative should write to the Chair of FIPAT for permission to publish a derivative work. Caput II: OSSA Chapter 2: BONES Latin term Latin synonym UK English US English English synonym Other 351 Systemata Musculoskeletal Musculoskeletal musculoskeletalia systems systems -

Nerve Activates Contraction
Joints Articulations of bones Functions of joints Hold bones together Allow for mobility Various kinds of joints. Fibrous: A, syndesmosis (tibiofibular); B, suture (skull). Cartilaginous: C, symphysis (vertebral bodies); D, synchondrosis (first rib and sternum). Synovial: E, condyloid (wrist); F, gliding (radioulnar); G, hinge or ginglymus (elbow); H, ball and socket (hip); I, saddle (carpometacarpal of thumb); J, pivot (atlantoaxial). Classification of Joints Functional Structural Synarthroses Fibrous joints Immovable joints Generally immovable Amphiarthroses Cartilaginous joints Slightly moveable Immovable or slightly joints moveable Diarthroses Synovial joints Freely moveable joints Freely moveable Fibrous Joints: Synarthrosis Syndesmoses Bones joined by fibrous bands (ligaments) Allows more movement than sutures Bones united by fibrous Example: Distal end of tissue tibia/fibula; radius/ ulna Bones fit tightly together Gomphosis Example: Fibrous tissue between tooth root and alveolar Sutures process of mandible or maxilla Teethlike projections of 2 bones interlock with thin No movement layer of fibrous tissue between them No movement Only in skull Cartilaginous Joints Bones connected by cartilage Example: Synchondrosis Hyaline cartilage between bones Slight movement Examples First rib and sternum Growth plate between epiphysis and diaphysis Symphysis Pad of fibrocartilage Slight movement Usually located along the midline of the body Pubic symphysis Between bodies of adjacent vertebrae Synovial -

Cartilaginous Joints*
OpenStax-CNX module: m46381 1 Cartilaginous Joints* OpenStax This work is produced by OpenStax-CNX and licensed under the Creative Commons Attribution License 3.0 Abstract By the end of this section, you will be able to: • Describe the structural features of cartilaginous joints • Distinguish between a synchondrosis and symphysis • Give an example of each type of cartilaginous joint As the name indicates, at a cartilaginous joint, the adjacent bones are united by cartilage, a tough but exible type of connective tissue. These types of joints lack a joint cavity and involve bones that are joined together by either hyaline cartilage or brocartilage (Figure 1 (Cartiliginous Joints )). There are two types of cartilaginous joints. A synchondrosis is a cartilaginous joint where the bones are joined by hyaline cartilage. Also classied as a synchondrosis are places where bone is united to a cartilage structure, such as between the anterior end of a rib and the costal cartilage of the thoracic cage. The second type of cartilaginous joint is a symphysis, where the bones are joined by brocartilage. *Version 1.3: Jun 4, 2013 10:16 am -0500 http://creativecommons.org/licenses/by/3.0/ http://cnx.org/content/m46381/1.3/ OpenStax-CNX module: m46381 2 Cartiliginous Joints Figure 1: At cartilaginous joints, bones are united by hyaline cartilage to form a synchondrosis or by brocartilage to form a symphysis. (a) The hyaline cartilage of the epiphyseal plate (growth plate) forms a synchondrosis that unites the shaft (diaphysis) and end (epiphysis) of a long bone and allows the bone to grow in length. -
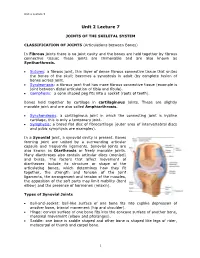
Joints of the Skeletal System
Unit 2 Lecture 5 Unit 2 Lecture 7 JOINTS OF THE SKELETAL SYSTEM CLASSIFICATION OF JOINTS (Articulations between Bones) In Fibrous joints there is no joint cavity and the bones are held together by fibrous connective tissue; these joints are Immovable and are also known as Syntharthrosis. Sutures: a fibrous joint, thin layer of dense fibrous connective tissue that unites the bones of the skull; becomes a synostosis in adult (by complete fusion of bones across joint. Syndesmosis: a fibrous joint that has more fibrous connective tissue (example is joint between distal articulation of tibia and fibula). Gomphosis: a cone shaped peg fits into a socket (roots of teeth). Bones held together by cartilage in cartilaginous joints. These are slightly movable joint and are also called Amphiarthrosis. Synchondrosis: a cartilaginous joint in which the connecting joint is hyaline cartilage, this is only a temporary joint. Symphysis: a broad flat disc of fibrocartilage (outer area of intervertebral discs and pubic symphysis are examples). In a Synovial joint, a synovial cavity is present. Bones forming joint are united by a surrounding articular capsule and frequently ligaments. Synovial joints are also known as Diarthrosis or freely movable joints. Many diarthroses also contain articular discs (menisci) and bursa. The factors that affect movement of diarthroses include its structure or shape of the articulating bones, which determines how they fit together, the strength and tension of the joint ligaments, the arrangement and tension of the muscles, the apposition of the soft parts may limit mobility (bent elbow) and the presence of hormones (relaxin). Types of Synovial Joints Ball-and-socket: ball-like surface of one bone fits into cuplike depression of another bone, triaxial movement (hip and shoulder).