Biochemistry of Central Nervous System (CNS) Blood-Brain Barrier (BBB)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Stems for Nonproprietary Drug Names
USAN STEM LIST STEM DEFINITION EXAMPLES -abine (see -arabine, -citabine) -ac anti-inflammatory agents (acetic acid derivatives) bromfenac dexpemedolac -acetam (see -racetam) -adol or analgesics (mixed opiate receptor agonists/ tazadolene -adol- antagonists) spiradolene levonantradol -adox antibacterials (quinoline dioxide derivatives) carbadox -afenone antiarrhythmics (propafenone derivatives) alprafenone diprafenonex -afil PDE5 inhibitors tadalafil -aj- antiarrhythmics (ajmaline derivatives) lorajmine -aldrate antacid aluminum salts magaldrate -algron alpha1 - and alpha2 - adrenoreceptor agonists dabuzalgron -alol combined alpha and beta blockers labetalol medroxalol -amidis antimyloidotics tafamidis -amivir (see -vir) -ampa ionotropic non-NMDA glutamate receptors (AMPA and/or KA receptors) subgroup: -ampanel antagonists becampanel -ampator modulators forampator -anib angiogenesis inhibitors pegaptanib cediranib 1 subgroup: -siranib siRNA bevasiranib -andr- androgens nandrolone -anserin serotonin 5-HT2 receptor antagonists altanserin tropanserin adatanserin -antel anthelmintics (undefined group) carbantel subgroup: -quantel 2-deoxoparaherquamide A derivatives derquantel -antrone antineoplastics; anthraquinone derivatives pixantrone -apsel P-selectin antagonists torapsel -arabine antineoplastics (arabinofuranosyl derivatives) fazarabine fludarabine aril-, -aril, -aril- antiviral (arildone derivatives) pleconaril arildone fosarilate -arit antirheumatics (lobenzarit type) lobenzarit clobuzarit -arol anticoagulants (dicumarol type) dicumarol -

A Graph Convolutional Network Framework for Predicting Multi-Type Drug-Drug Interactions
MTDDI: a graph convolutional network framework for predicting Multi-Type Drug-Drug Interactions YueHua Feng ( [email protected] ) Northwestern Polytechnical University https://orcid.org/0000-0002-3783-1305 Shao-Wu Zhang Northwestern Polytechnical University https://orcid.org/0000-0003-1305-7447 Qing-Qing Zhang Northwestern Polytechnical University https://orcid.org/0000-0002-7931-1834 Chu-Han Zhang Northwestern Polytechnical University https://orcid.org/0000-0002-2897-3918 Jian-Yu Shi Northwestern Polytechnical University https://orcid.org/0000-0002-2303-273X Research article Keywords: Drug-drug interactions (DDIs), multi-type DDIs prediction, graph convolution network (GCN), tensor factorization, deep neural network, multiple relation prediction, similarity regularization Posted Date: April 9th, 2021 DOI: https://doi.org/10.21203/rs.3.rs-397281/v1 License: This work is licensed under a Creative Commons Attribution 4.0 International License. Read Full License MTDDI: a graph convolutional network framework for predicting Multi-Type Drug-Drug Interactions Yue-Hua Feng1, Shao-Wu Zhang1*, Qing-Qing Zhang1, Chu-Han Zhang2, Jian-Yu Shi3* 1 Key Laboratory of Information Fusion Technology of Ministry of Education, School of Automation, Northwestern Polytechnical University, Xi’an, 710072, China 2 School of Software, Northwestern Polytechnical University, Xian, 710072, China 3 School of Life Sciences, Northwestern Polytechnical University, Xi’an, 710072, China * Correspondence: [email protected] ; [email protected] Abstract— Although the polypharmacy has both higher therapeutic efficacy and less drug resistance in combating complex diseases, drug-drug interactions (DDIs) may trigger unexpected pharmacological effects, such as side effects, adverse reactions, or even serious toxicity. Thus, it is crucial to identify DDIs and explore its underlying mechanism (e.g., DDIs types) for polypharmacy safety. -

Les Femmes Peuvent Prendre Du Viagra * Achat De Viagra En
Viagra est indiquée pour le traitement de la dysfonction érectile masculine. >>> ORDER NOW <<< Les femmes peuvent prendre du viagra Tags: cialis ou viagra acheter vrai ou faux viagra risques avec viagra acheter du viagra sans ordonnance en suisse danger du faux viagra comment bien prendre viagra le prix du viagra en pharmacie au maroc les effets indesirable du viagra que ce que viagra de gaulle contre le viagra achat viagra internet doctissimo commande viagra belgique peut on avoir du viagra en pharmacie sans ordonnance comment prendre sildenafil pfizer nitrates and viagra interaction ordonnance ou pas pour viagra notice demballage viagran quels sont les effets du viagra quelle quantité de viagra prendre peut on acheter viagra en pharmacie sans ordonnance comment avoir le viagra site sur achat viagra nitrates viagra can deadly combination Recession quest ce qui peut remplacer le viagra review am astonishingly forgetful. Some things said caffeine was fine to consume (in drinks, food, etc) while on the med, others said caffeine decreased the effects of the med. Features of this disorder include dysphonia, dysarthria, and loss of pain and temperature over the ipsilateral face and contralateral body. Jeg vil også erklære, at du forlader soap ud. If you feel very bored when waiting for something or someone (a bus, your friend, your kids), distract yourself with a book, magazine, or crossword puzzle. The celexa is longer acting and must build in the system over time in order to work for anxiety. Medscape is the leading online destination for healthcare professionals seeking clinical information. Paxil over time increased my appetite but i think that is because les femmes peuvent prendre du viagra made me tired and lethargic, they did not increase appetite, in fact in the first few months they curbed it right down. -

The Use of Stems in the Selection of International Nonproprietary Names (INN) for Pharmaceutical Substances
WHO/PSM/QSM/2006.3 The use of stems in the selection of International Nonproprietary Names (INN) for pharmaceutical substances 2006 Programme on International Nonproprietary Names (INN) Quality Assurance and Safety: Medicines Medicines Policy and Standards The use of stems in the selection of International Nonproprietary Names (INN) for pharmaceutical substances FORMER DOCUMENT NUMBER: WHO/PHARM S/NOM 15 © World Health Organization 2006 All rights reserved. Publications of the World Health Organization can be obtained from WHO Press, World Health Organization, 20 Avenue Appia, 1211 Geneva 27, Switzerland (tel.: +41 22 791 3264; fax: +41 22 791 4857; e-mail: [email protected]). Requests for permission to reproduce or translate WHO publications – whether for sale or for noncommercial distribution – should be addressed to WHO Press, at the above address (fax: +41 22 791 4806; e-mail: [email protected]). The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the World Health Organization concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. Dotted lines on maps represent approximate border lines for which there may not yet be full agreement. The mention of specific companies or of certain manufacturers’ products does not imply that they are endorsed or recommended by the World Health Organization in preference to others of a similar nature that are not mentioned. Errors and omissions excepted, the names of proprietary products are distinguished by initial capital letters. -

Pharmaceutical Appendix to the Tariff Schedule 2
Harmonized Tariff Schedule of the United States (2007) (Rev. 2) Annotated for Statistical Reporting Purposes PHARMACEUTICAL APPENDIX TO THE HARMONIZED TARIFF SCHEDULE Harmonized Tariff Schedule of the United States (2007) (Rev. 2) Annotated for Statistical Reporting Purposes PHARMACEUTICAL APPENDIX TO THE TARIFF SCHEDULE 2 Table 1. This table enumerates products described by International Non-proprietary Names (INN) which shall be entered free of duty under general note 13 to the tariff schedule. The Chemical Abstracts Service (CAS) registry numbers also set forth in this table are included to assist in the identification of the products concerned. For purposes of the tariff schedule, any references to a product enumerated in this table includes such product by whatever name known. ABACAVIR 136470-78-5 ACIDUM LIDADRONICUM 63132-38-7 ABAFUNGIN 129639-79-8 ACIDUM SALCAPROZICUM 183990-46-7 ABAMECTIN 65195-55-3 ACIDUM SALCLOBUZICUM 387825-03-8 ABANOQUIL 90402-40-7 ACIFRAN 72420-38-3 ABAPERIDONUM 183849-43-6 ACIPIMOX 51037-30-0 ABARELIX 183552-38-7 ACITAZANOLAST 114607-46-4 ABATACEPTUM 332348-12-6 ACITEMATE 101197-99-3 ABCIXIMAB 143653-53-6 ACITRETIN 55079-83-9 ABECARNIL 111841-85-1 ACIVICIN 42228-92-2 ABETIMUSUM 167362-48-3 ACLANTATE 39633-62-0 ABIRATERONE 154229-19-3 ACLARUBICIN 57576-44-0 ABITESARTAN 137882-98-5 ACLATONIUM NAPADISILATE 55077-30-0 ABLUKAST 96566-25-5 ACODAZOLE 79152-85-5 ABRINEURINUM 178535-93-8 ACOLBIFENUM 182167-02-8 ABUNIDAZOLE 91017-58-2 ACONIAZIDE 13410-86-1 ACADESINE 2627-69-2 ACOTIAMIDUM 185106-16-5 ACAMPROSATE 77337-76-9 -
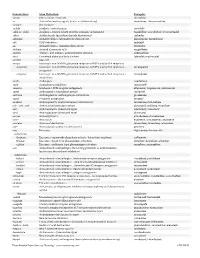
Downloaded from Survive Nursing | Survivenursing.Com V20110426
Generic Stem Stem Definition Examples -abine (see -arabine, -citabine) decitabine -ac Anti-inflammatory agents (acetic acid derivatives) bromfenac; dexpemedolac -acetam See -racetam -actide Synthetic corticotropins seractide -adol or -aldol- Analgesics (mixed opiate receptor agonists/ antagonists) tazadolene; spiradolene; levonantradol -adox Antibacterials (quinoline dioxide derivatives) carbadox -afenone Antiarrhythmics (propafenone derivatives) alprafenone; diprafenone -afil PDE5 inhibitors tadalafil -aj- Antiarrhythmics (ajmaline derivatives) lorajmine -aldrate Antacid aluminum salts magaldrate -algron Alpha1 - and alpha2 - adrenoreceptor agonists dabuzalgron -alol Combined alpha and beta blockers labetalol; medroxalol -amivir (see -vir) -ampa Ionotropic non-NMDA glutamate receptors (AMPA and/or KA receptors) -ampanel Ionotropic non-NMDA glutamate receptors (AMPA and/or KA receptors) ; becampanel antagonists -ampator Ionotropic non-NMDA glutamate receptors (AMPA and/or KA receptors) ; forampator modulators -andr- Androgens nandrolone -anib Angiogenesis inhibitors semaxanib -anserin Serotonin 5-HT2 receptor antagonists altanserin; tropanserin; adatanserin -antel Anthelmintics (undefined group) carbantel -antrone Antineoplastics; anthraquinone derivatives pixantrone -apsel P-selectin antagonists torapsel -arabine Antineoplastics (arabinofuranosyl derivatives) fazarabine; fludarabine aril-, -aril, -aril- Antiviral (arildone derivatives) pleconaril; arildone; fosarilate -arit Antirheumatics (lobenzarit type) lobenzarit; clobuzarit -arol -

Marrakesh Agreement Establishing the World Trade Organization
No. 31874 Multilateral Marrakesh Agreement establishing the World Trade Organ ization (with final act, annexes and protocol). Concluded at Marrakesh on 15 April 1994 Authentic texts: English, French and Spanish. Registered by the Director-General of the World Trade Organization, acting on behalf of the Parties, on 1 June 1995. Multilat ral Accord de Marrakech instituant l©Organisation mondiale du commerce (avec acte final, annexes et protocole). Conclu Marrakech le 15 avril 1994 Textes authentiques : anglais, français et espagnol. Enregistré par le Directeur général de l'Organisation mondiale du com merce, agissant au nom des Parties, le 1er juin 1995. Vol. 1867, 1-31874 4_________United Nations — Treaty Series • Nations Unies — Recueil des Traités 1995 Table of contents Table des matières Indice [Volume 1867] FINAL ACT EMBODYING THE RESULTS OF THE URUGUAY ROUND OF MULTILATERAL TRADE NEGOTIATIONS ACTE FINAL REPRENANT LES RESULTATS DES NEGOCIATIONS COMMERCIALES MULTILATERALES DU CYCLE D©URUGUAY ACTA FINAL EN QUE SE INCORPOR N LOS RESULTADOS DE LA RONDA URUGUAY DE NEGOCIACIONES COMERCIALES MULTILATERALES SIGNATURES - SIGNATURES - FIRMAS MINISTERIAL DECISIONS, DECLARATIONS AND UNDERSTANDING DECISIONS, DECLARATIONS ET MEMORANDUM D©ACCORD MINISTERIELS DECISIONES, DECLARACIONES Y ENTEND MIENTO MINISTERIALES MARRAKESH AGREEMENT ESTABLISHING THE WORLD TRADE ORGANIZATION ACCORD DE MARRAKECH INSTITUANT L©ORGANISATION MONDIALE DU COMMERCE ACUERDO DE MARRAKECH POR EL QUE SE ESTABLECE LA ORGANIZACI N MUND1AL DEL COMERCIO ANNEX 1 ANNEXE 1 ANEXO 1 ANNEX -

Federal Register / Vol. 60, No. 80 / Wednesday, April 26, 1995 / Notices DIX to the HTSUS—Continued
20558 Federal Register / Vol. 60, No. 80 / Wednesday, April 26, 1995 / Notices DEPARMENT OF THE TREASURY Services, U.S. Customs Service, 1301 TABLE 1.ÐPHARMACEUTICAL APPEN- Constitution Avenue NW, Washington, DIX TO THE HTSUSÐContinued Customs Service D.C. 20229 at (202) 927±1060. CAS No. Pharmaceutical [T.D. 95±33] Dated: April 14, 1995. 52±78±8 ..................... NORETHANDROLONE. A. W. Tennant, 52±86±8 ..................... HALOPERIDOL. Pharmaceutical Tables 1 and 3 of the Director, Office of Laboratories and Scientific 52±88±0 ..................... ATROPINE METHONITRATE. HTSUS 52±90±4 ..................... CYSTEINE. Services. 53±03±2 ..................... PREDNISONE. 53±06±5 ..................... CORTISONE. AGENCY: Customs Service, Department TABLE 1.ÐPHARMACEUTICAL 53±10±1 ..................... HYDROXYDIONE SODIUM SUCCI- of the Treasury. NATE. APPENDIX TO THE HTSUS 53±16±7 ..................... ESTRONE. ACTION: Listing of the products found in 53±18±9 ..................... BIETASERPINE. Table 1 and Table 3 of the CAS No. Pharmaceutical 53±19±0 ..................... MITOTANE. 53±31±6 ..................... MEDIBAZINE. Pharmaceutical Appendix to the N/A ............................. ACTAGARDIN. 53±33±8 ..................... PARAMETHASONE. Harmonized Tariff Schedule of the N/A ............................. ARDACIN. 53±34±9 ..................... FLUPREDNISOLONE. N/A ............................. BICIROMAB. 53±39±4 ..................... OXANDROLONE. United States of America in Chemical N/A ............................. CELUCLORAL. 53±43±0 -

| Hao Wanat Ha Maria Del Mar Man Unit Minit
|HAO WANAT HA MARIAUS009731026B2 DEL MAR MAN UNIT MINIT (12 ) United States Patent ( 10 ) Patent No. : US 9 ,731 , 026 B2 Su et al. (45 ) Date of Patent: Aug. 15 , 2017 ( 54 ) NEAT LIQUID PHARMACEUTICAL (58 ) Field of Classification Search FORMULATIONS ??? . .. A61K 8 /02 See application file for complete search history . @( 71) Applicant : Massachusetts Institute of Technology , Cambridge, MA (US ) (56 ) References Cited U . S . PATENT DOCUMENTS @( 72 ) Inventors : Erzheng Su , Cambridge, MA (US ) ; Alexander M . Klibanov , Boston , MA 3 , 800 ,038 A 3 / 1974 Rudel ( US ) 5 ,405 ,617 A 4 / 1995 Gowan 7 , 763 ,653 B2 7 / 2010 Pacheco @( 73 ) Assignee : Massachusetts Institute of 2009/ 0004281 Al * 1 / 2009 Nghiem . .. .. .. .. A61K 9 / 0004 Technology , Cambridge , MA (US ) 424 /490 @( * ) Notice : Subject to any disclaimer , the term of this OTHER PUBLICATIONS patent is extended or adjusted under 35 U . S . C . 154 ( b ) by 0 days . Abbott , et al ., “ Novel solvent properties of choline chloride /urea mixtures " , Chem . Commun . , 1 : 70 - 71 ( 2003 ) . Abbott, et al. , “ Ionic liquid analogues formed from hydrated metal ( 21 ) Appl. No. : 14 / 951, 055 salts ” , Chem . , Eur. J . , 10 : 3769 -74 ( 2004 ) . Bica , et al ., “ Liquid forms of pharmaceutical co -crystals : Exploring (22 ) Filed : Nov . 24 , 2015 the boundaries of salt formation ” , Chem Comm ., 47 ( 8 ) : 2267- 9 ( 2011 ) . (65 ) Prior Publication Data Grodowska , et al. , “ Organic solvents in the pharmaceutical indus US 2016 / 0143848 A1 May 26 , 2016 try ” , Acta Polomine Pharma. , 67 ( 1 ) : 3 - 12 (2010 ) . International Search Report for Corresponding PCT/ US2015 / Related U . S . Application Data 062470 mailed Feb . 3 , 2016 . -

Stembook 2018.Pdf
The use of stems in the selection of International Nonproprietary Names (INN) for pharmaceutical substances FORMER DOCUMENT NUMBER: WHO/PHARM S/NOM 15 WHO/EMP/RHT/TSN/2018.1 © World Health Organization 2018 Some rights reserved. This work is available under the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 IGO licence (CC BY-NC-SA 3.0 IGO; https://creativecommons.org/licenses/by-nc-sa/3.0/igo). Under the terms of this licence, you may copy, redistribute and adapt the work for non-commercial purposes, provided the work is appropriately cited, as indicated below. In any use of this work, there should be no suggestion that WHO endorses any specific organization, products or services. The use of the WHO logo is not permitted. If you adapt the work, then you must license your work under the same or equivalent Creative Commons licence. If you create a translation of this work, you should add the following disclaimer along with the suggested citation: “This translation was not created by the World Health Organization (WHO). WHO is not responsible for the content or accuracy of this translation. The original English edition shall be the binding and authentic edition”. Any mediation relating to disputes arising under the licence shall be conducted in accordance with the mediation rules of the World Intellectual Property Organization. Suggested citation. The use of stems in the selection of International Nonproprietary Names (INN) for pharmaceutical substances. Geneva: World Health Organization; 2018 (WHO/EMP/RHT/TSN/2018.1). Licence: CC BY-NC-SA 3.0 IGO. Cataloguing-in-Publication (CIP) data. -

Appendix E-3: Complete Search Strategy
1 Appendix e-3: Complete search strategy The authors initially performed database searches for all studies published in regard to cognitive and emotional disorders in multiple sclerosis. The final guideline focuses solely on emotional disorders. The search strategy presents all results pertaining to emotional disorders unless a search included data on both cognitive and emotional disorders with respect to a particular topic (e.g., interventions). Medline search strategy While the staff of HealthSearch makes every effort to ensure that the information gathered is accurate and up-to-date, HealthSearch disclaims any warranties regarding the accuracy or completeness of the information or its fitness for a particular purpose. HealthSearch provides information from public sources both in electronic and print formats and does not guarantee its accuracy, completeness or reliability. The information provided is only for the use of the Client and no liability is accepted by HealthSearch to third parties. Database: Ovid MEDLINE(R) <1950 to February Week 1 2007> Search Strategy: -------------------------------------------------------------------------------- 1 multiple sclerosis/ or multiple sclerosis, chronic progressive/ or multiple sclerosis, relapsing- remitting/ or neuromyelitis optica/ (28475) 2 Demyelinating Diseases/ (7686) 3 clinically isolated syndrome.mp. (67) 4 first demyelinating event.mp. (23) 5 multiple sclerosis.mp. (33263) 6 Demyelinating Disease:.mp. (9613) 7 disseminated sclerosis.mp. (491) 8 or/1-7 (40459) 9 affective symptoms/ (8380) 10 emotions/ or affect/ or irritable mood/ or exp anxiety/ (70058) 11 mood disorders/ or affective disorders, psychotic/ or bipolar disorder/ or cyclothymic disorder/ or depressive disorder/ or depression, postpartum/ or depressive disorder, major/ or dysthymic disorder/ or seasonal affective disorder/ (73853) 12 mood swing:.mp. -

(12) Patent Application Publication (10) Pub. No.: US 2013/0065861 A1 Wu Et Al
US 2013 OO65861A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2013/0065861 A1 Wu et al. (43) Pub. Date: Mar. 14, 2013 (54) COMPOUNDS (CYSTEIN BASED Publication Classification LIPOPEPTIDES) AND COMPOSITIONS AS (51) Int. Cl. TLR2AGONSTS USED FORTREATING A63L/23 (2006.01) INFECTIONS, INFLAMMATIONS, A6IP II/06 (2006.01) RESPRATORY DISEASESETC. A6IP II/00 (2006.01) A6IPL/04 (2006.01) A6IPI/00 (2006.01) A6IP35/00 (2006.01) (75) Inventors: Tom Yao-Hsiang Wu, San Diego, CA A6IP 9/02 (2006.01) (US); Yefen Zou, San Diego, CA (US); C07F 9/40 (2006.01) Timothy Z. Hoffman, San Diego, CA A6IP3 L/18 (2006.01) (US); Jianfeng Pan, San Diego, CA A63/662 (2006.01) C07D 213/56 (2006.01) (US) A613 L/4402 (2006.01) C07C 237/06 (2006.01) C07D 265/30 (2006.01) (73) Assignee: IRM LLC, Hamiltona (BM) A 6LX3/5.375 (2006.01) C07D 233/6 (2006.01) (21) Appl. No.: 13/636,328 A613 L/4164 (2006.01) C07C 237/04 (2006.01) PCT Fled: Mar. 23, 2011 (52) U.S. Cl. (22) USPC ........... 514/119:554/101: 514/548; 546/335; 514/357: 514/547: 544/159; 514/237.8: 548/338.5; (86) PCT NO.: PCT/US11A29661 514/399 S371 (c)(1), (57) ABSTRACT (2), (4) Date: Sep. 20, 2012 The invention provides a novel class of compounds viz. gen erally lipopeptides like Pam3CSK4, immunogenic composi tions and pharmaceutical compositions comprising Such Related U.S. Application Data compounds and methods of using such compounds to treat or prevent diseases or disorders associated with Toll-Like (60) Provisional application No.