Comparison of Automated Hearing Testing Approaches for Outpatients Receiving Ototoxic Chemotherapy
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

OMS II Medical Skills Competencies
SOMA OMS II Medical Skills Competencies Exam Checklist #1 Performed Performed Did not satisfactorily less than perform General, Vital Signs, Skin, Hair, and Nails (1 point) satisfactorily (0 point) (0.5 point) GENERAL 1. Introduces self to patient including full name and status (2nd year osteopathic medical student). 2. Demonstrates respect in addressing patient. 3. Washes hands before shaking hands. 4. Washes hands before beginning physical exam. VITAL SIGNS 5. Takes radial pulse rate. 6. Palpates radial pulse bilaterally. 7. Takes respiratory rate. 8. Takes BP, patient seated comfortably, feet on floor, cuff at heart level and arm supported. 9. Explains vital sign results to patient. SKIN 10. Inspects entire skin surface, explaining reason for inspection to patient 11. Demonstrates proper draping to preserve patient modesty. 12. Palpates skin for turgor, texture, and capillary refill. HAIR 13. Notes observed hair distribution on scalp and body. When possible and appropriate, includes pelvic hair distribution. 14. Describes scalp hair color, distribution and character (thick, thin, straight, curly). NAILS 15. Describes nail abnormalities- color, shape, texture, thickness (lines, brittleness, thickening, discoloration, pitting, ridges, spooning) Score ___/15 Student Name: ________________________________ Student Name: _____________________ Community Campus: ________________________________ Signature of Preceptor: _________________________ Name of Preceptor:_______________________ Date of Completion: ______________ Form Updated 7/10/15 SOMA OMS II Medical Skills Competencies Physical Exam Checklist #2: Performed Performed less Did not perform Head, Eyes, CN’s II-XII satisfactorily than (0 point) (1 point) satisfactorily (0.5 point) EYES 1. Visual Acuity (CN II.) 2. Visual Fields to confrontation 3. Extraocular Movements: (CNs - III, IV, VI.) 4. -

An Evaluation of Two Pulse-Type Tests Oe Hearing
AN EVALUATION OF TWO PULSE-TYPE TESTS OE HEARING Dissertation Presented in Partial Fulfillment of the Requirements for the Degree Doctor of Philosophy in the Graduate School of The Ohio State University By THOMAS BROWN ANDERSON, B.S., M. S. The Ohio State University 1952 Approved by: TABLE OE CONTENTS CHAPTER I. INTRODUCTION .... ............. 1 The Problem...................... 7 The Hypotheses ................. 8 Explanation of Terms . ........ 12 II. REVIEW OE LITERATURE ............. 14 III. PROCEDURES........................ 26 Apparatus........................ 26 Stimuli................... .. 28 Subjects ........................ 29 Presentation ................... 30 Scoring of Tests ............... 33 IV. RESULTS AND DISCUSSION........... 34 Part I .......................... 34 Part II ........................ 96 V. CONCLUSIONS ...................... 99 BIBLIOGRAPHY............................ 104 APPENDIX A ............................... 108 APPENDIX B ............................... 110 APPENDIX C............................... 111 APPENDIX D . ............................ 112 APPENDIX E ............................... 113 APPENDIX E ............................... 114 AUTOBIOGRAPHY .......................... 115 ii E09365 LIST OE TABLES TABLE PAGE I. Order of Tests for Each. Group of 10 Subjects . 27 II. Analyses Made and Statistical Methods Used . 36 III. Summary of Tests of Independence and Correla tion of Pure-Tone Scores and Speech- Reception Scores ...................... 38 IV. Summary of Eight Analyses of Variance: Measures -

Hearing Screening Training Manual REVISED 12/2018
Hearing Screening Training Manual REVISED 12/2018 Minnesota Department of Health (MDH) Community and Family Health Division Maternal and Child Health Section 1 2 For more information, contact Minnesota Department of Health Maternal Child Health Section 85 E 7th Place St. Paul, MN 55164-0882 651-201-3760 [email protected] www.health.state.mn.us Upon request, this material will be made available in an alternative format such as large print, Braille or audio recording. 3 Revisions made to this manual are based on: Guidelines for Hearing Screening After the Newborn Period to Kindergarten Age http://www.improveehdi.org/mn/library/files/afternewbornperiodguidelines.pdf American Academy of Audiology, Childhood Screening Guidelines http://www.cdc.gov/ncbddd/hearingloss/documents/AAA_Childhood%20Hearing%2 0Guidelines_2011.pdf American Academy of Pediatrics (AAP), Hearing Assessment in Children: Recommendations Beyond Neonatal Screening http://pediatrics.aappublications.org/content/124/4/1252 4 Contents Introduction .................................................................................................................... 7 Audience ..................................................................................................................... 7 Purpose ....................................................................................................................... 7 Overview of hearing and hearing loss ............................................................................ 9 Sound, hearing, and hearing -

Audiometric Test Procedures
Audiometric Test Procedures 101 This information is meant to help you better understand the various test procedures as well as some of the terms you might see on an audiometric report. By Larry Medwetsky individual could, in fact, exhibit nor- In the previous issue of Hearing mal hearing acuity across these three Loss Magazine, I provided an over- Anyone who has ever had their frequencies, yet, exhibit a significant view concerning hearing threshold hearing tested should know how hearing loss in the higher frequencies results as recorded on the audiogram to read the audiogram, but that’s (3000-8000 Hz). Thus, it is important and an explanation of the pure-tone easier said than done. Hopefully, to examine the SRT in the context of audiogram. In this article, I will after reading this article you will the other audiometric test findings. describe various test procedures have a greater understanding of the Speech Awareness Threshold that are typically administered in principles discussed and use your (SAT): an audiometric evaluation and what knowledge going forward—be it in Compound words are pre- information the tests provide. reviewing hearing test results you sented, the goal being to determine already have or when discussing your the softest level one can detect the Audiometric Test Procedures results at your next hearing test. presence of words. This test is often Pure-tone Audiometry: Tones of used when an individual’s hearing loss different frequencies are presented; the is so great that the person is unable goal is to find the softest sound level relatively flat hearing losses, and the to recognize/repeat the words, yet is which one can hear (threshold) the average of 500 and 1000 Hz for those aware that words have been presented. -

File Code: 5100/6180 Date: February, 2020
United States Forest Washington Office of Department of Service Fire & Aviation Agriculture File Code: 5100/6180 Date: February, 2020 eOF-178 Medical Exam GUIDANCE SHEET for the Medical Provider **ADDITIONAL ANCILLARY TESTS beyond what is on the OF-178 form are not approved and will not be covered by the Forest Service** eMedical Access Directions – 1. Access eMedical here. Please bookmark this site for all future use. https://emedicalacc.gdcii.com/provider 2. If you are new to eMedical, click on “Establish Username and Password” and create a profile. This information is confidential and is used to digitally sign/submit the exam. 3. Ensure that the provider’s eMedical profile includes the examining medical provider’s information, as this is what is used to create the exam’s electronic signature. 4. Once logged in, click on “Redeem Invitation” and enter the patient exam “Invitation” code. Then verify patient DOB. 5. The patient exam “Invitation” code is a 16 digit alphanumeric code. a. The code will be provided by the patient when they come to their exam. On employee provided emails it is found under Step 6 of the Medical Provider Use Only section. 6. Once the code is claimed, click on “My Packets”, then “Take Action” to complete and submit the exam. You may log in/out as many times as you need. The exam will be under “My Packets” until it is submitted. 7. For further information, you may visit the USFS eMedical website here: https://www.fs.fed.us/managing-land/fire/safety/emedical NEED HELP? Email your name, contact info (email/phone) and the employee you need assistance with to [email protected] or call 208-387-5628 and we will assist you ASAP. -
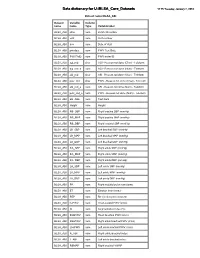
BLSA Data Dictionary
Data dictionary for U:\BLSA_Core_Datasets 11:15 Tuesday, January 2, 2018 Dataset name=BLSA_ABI Dataset Variable Column name name Type Variable label BLSA_ABI idno num BLSA ID number BLSA_ABI visit num Visit number BLSA_ABI dov num Date of Visit BLSA_ABI pwvdate num PWV Test Date BLSA_ABI PWVTtrID num PWV tester ID BLSA_ABI agi_rnd char AGI - Reason not done (Char) - Teleform BLSA_ABI agi_rnd_a num AGI - Reason not done (Num) - Teleform BLSA_ABI abi_rnd char ABI - Reason not done (Char) - Teleform BLSA_ABI pwv_rnd char PWV - Reason not done (Char) - Teleform BLSA_ABI abi_rnd_a num ABI - Reason not done (Num) - Teleform BLSA_ABI pwv_rnd_a num PWV - Reason not done (Num) - Teleform BLSA_ABI abi_date num Test Date BLSA_ABI Height num Height BLSA_ABI RB_SBP num Right brachia SBP (mmHg) BLSA_ABI RB_MAP num Right brachia MAP (mmHg) BLSA_ABI RB_DBP num Right brachial SBP (mmHg) BLSA_ABI LB_SBP num Left brachial SBP (mmHg) BLSA_ABI LB_MAP num Left brachial MAP (mmHg) BLSA_ABI LB_DBP num Left brachial DBP (mmHg) BLSA_ABI RA_SBP num Right ankle SBP (mmHg) BLSA_ABI RA_MAP num Right ankle MAP (mmHg) BLSA_ABI RA_DBP num Right ankle DBP (mmHg) BLSA_ABI LA_SBP num Left ankle SBP (mmHg) BLSA_ABI LA_MAP num Left ankle MAP (mmHg) BLSA_ABI LA_DBP num Left ankle DBP (mmHg) BLSA_ABI PR num Right brachial pulse rate (bpm) BLSA_ABI ET num Ejection time (msec) BLSA_ABI PEP num Re ejection period (msec) BLSA_ABI hcPWV num Heart-carotid PWV (cm/s) BLSA_ABI AI num Augmentation index (%) BLSA_ABI RhbPWV num Heart-brachial PWV (cm/s) BLSA_ABI RbaPWV num Right ankle -

Therapy Early Referral Primary Healtllcare Workers Project
ISSN 1368-2105 ISSN (online) 2045-6174 www.speechmag.com Enjoy your SpeecliMMER 1997 meal! Use of th ickener in guage dysphagia Fluency - Promoling Therapy early referral Primary HealtllCare Workers Project Focus on Your clinical companion Derwen Objective setting in a specialist Trust Minimal.ly responsive state A team approach to severe brain injury How I ... Illanage dysartflria Three tllerapists dis uss a case Naming - more than just right tiv or wrong? Responses to cueing in aphasia Information for I rod clng WILSTAAR in contributors p om Common queries an wered s ion Is. ISSN (online) 2045-6174 www.speechmag.com The big breakthrough on a small scale he new DynaMyte is a lightweight, Tportable device with powerful communication capabilities which introduces a new dimension to augmentative communication by offering greater freedom to the ambulant user. DynaMyte is just half the size of DynaVox 2 and yet it retains all its advanced communication capabilities, and uses the same software. A built-in remote control unit allows the user to access computers and other household appliances, and it The DynaVox 2 augmentative features a system of alarms capable of performing a variety communication aid of preset tasks. A clear, easy to operate touch display has introduced a provides access to the full range of DynaMyte's new era of freedom to people of a/l ages who communication power. Its long life battery and durable, have speech disabilities. rubberised casing guarantees easy to carry communication for people of all ages with speech disabilities. DynaMyte is a natural product extension from the advanced DynaVox 2 communication device which successfully enables many users with mobility impairment to develop a greater sense of self expression and independence. -

8 Hearing Measurement
8 HEARING MEASUREMENT John R. Franks, Ph.D. Chief, Hearing Loss Prevention Section Engineering and Physical Hazards Branch Division of Applied Research and Technology National Institute for Occupational Safety and Health Robert A. Taft Laboratories 4676 Columbia Parkway Cincinnati, Ohio 45226-1998 USA [email protected] 8.1. INTRODUCTION (RATIONALE FOR AUDIOMETRY) The audiogram is a picture of how a person hears at a given place and time under given conditions. The audiogram may be used to describe the hearing of a person for the various frequencies tested. It may be used to calculate the amount of hearing handicap a person has. And, it may be used as a tool to determine the cause of a person’s hearing loss. Audiograms may be obtained in many ways; e.g., by using pure tones via air conduction or bone conduction for behavioral testing or by using tone pips to generate auditory brainstem responses. The audiogram is a most unusual biometric test. It is often incorrectly compared to a vision test. In the audiogram, the goal is to determine the lowest signal level a person can hear. In the case of a vision test, the person reads the smallest size of print that he or she can see, the auditory equivalent of identifying the least perceptible difference between two sounds. In most occupational and medical settings, this requires the listener to respond to very low levels of sounds that he or she does not hear in normal day-to-day life. A vision test analogous to an audiogram would require a person to sit in a totally darkened room and be tested for the lowest luminosity light of various colors, red to blue, that can be seen. -

Ear Examination
Ear Examination Introduction Wash hands, Introduce self, Patients name & DOB & what they like to be called, Explain examination and get consent Explain procedure and that they must stay completely still when you use the otoscope Position patient – seat at same level as you with access to both ears Note and remove any hearing aids Get otoscope, speculum, 512Hz tuning fork Inspection General inspection: symmetry, position (low set = genetic syndromes), shape Close ear inspection: o Skin in front and behind ear: skin tags, erythema, scars, preauricular sinuses/pits o Pinna: any skin changes (e.g. neoplasia), deformities (e.g. accessory auricle), scars, erythema (erysipelas, chondritis), perichondrial haematoma (trauma) o External auditory meatus: erythema, pus/discharge (otitis externa) o Mastoid: erythema/swelling (mastoiditis) Palpation Tug pinna gently (tenderness = mastoiditis) Palpate mastoid (tenderness = mastoiditis) Feel for pre/post-auricular lymph nodes (infections) Otoscopy Apply speculum to otoscope and turn on light Hold the otoscope like a pencil (in your right hand for their right ear and vice versa) with the handle pointing anteriorly Start with non-affected ear Pull the pinna up and backwards (down and backwards in children) with your other hand to straighten the external auditory canal Insert the speculum tip into the external auditory meatus Rest the ulnar border of your hand on their cheek to stabilise it Gently advance the speculum while looking through the otoscope Look at o Auditory canal – look for: wax, foreign bodies, skin quality (thick white growth = cholesteatoma), erythema/discharge (otitis externa) o Tympanic membrane . Colour – should be pinkish-grey (red = infection; scarred = typanosclerosis) . Structure – look for perforation, tympanostomy (grommet), bulging (infection) or retraction (Eustachian tube dysfunction) . -

Evaluation of Malingering Characteristics and Strategies During Hearing Assessment Sarah Michelle Thompson
University of Northern Colorado Scholarship & Creative Works @ Digital UNC Capstones Student Research 5-2017 Evaluation of Malingering Characteristics and Strategies During Hearing Assessment Sarah Michelle Thompson Follow this and additional works at: http://digscholarship.unco.edu/capstones Recommended Citation Thompson, Sarah Michelle, "Evaluation of Malingering Characteristics and Strategies During Hearing Assessment" (2017). Capstones. 19. http://digscholarship.unco.edu/capstones/19 This Text is brought to you for free and open access by the Student Research at Scholarship & Creative Works @ Digital UNC. It has been accepted for inclusion in Capstones by an authorized administrator of Scholarship & Creative Works @ Digital UNC. For more information, please contact [email protected]. ©2017 SARAH MICHELLE THOMPSON ALL RIGHTS RESERVED UNIVERSITY OF NORTHERN COLORADO Greeley, Colorado The Graduate School EVALUATION OF MALINGERING CHARACTERISTICS AND STRATEGIES DURING HEARING ASSESSMENT A Capstone Research Project Submitted in Partial Fulfillment of the Requirements for the Degree of Doctor of Audiology Sarah Michelle Thompson College of Natural and Health Sciences School of Human Sciences Audiology & Speech-Language Sciences May 2017 This Capstone Project by: Sarah Michelle Thompson Entitled: Evaluation of Malingering Characteristics and Strategies During Hearing Assessment Has been approved as meeting the requirement for the Degree of Doctor of Audiology in the College of Natural and Health Sciences in the School of Human Sciences, Program of Audiology and Speech-Language Sciences Accepted by the Capstone Research Committee Kathryn Bright, Ph.D., Co-Research Advisor Tina M. Stoody, Ph.D., Co-Research Advisor Kathleen Fahey, Ph.D., Committee Member Accepted by the Graduate School Linda L. Black, Ed.D. Associate Provost and Dean Graduate School and International Admission ABSTRACT Thompson, Sarah Michelle. -

Hearing Testing Early
Otology & Neurotology 39:S30–S42 ß 2018, Otology & Neurotology, Inc. Assessment of Hearing During the Early Years of the American Otological Society Matthew B. Fitzgerald and Robert K. Jackler Department of Otolaryngology—Head and Neck Surgery, Stanford Ear Institute, Stanford University, Palo Alto, California Objective: To describe the manner in which hearing was with the distance a watch tick could be heard over the evaluated in American Otological Practice during the late distance of a normal hearing individual. A variety of devices, 19th and early 20th centuries before introduction of the such as Politzer’s Acoumeter, attempted to deliver sound in electric audiometer. a calibrated manner, thus enhancing the accuracy and Methods: Primary sources were the Transactions of the reproducibility of test results. American Otological Society and American textbooks, espe- Conclusion: The early years of the American Otological cially those authored by Presidents of the Society. Society were marked by a number of ingenious efforts to Results: In the era before electric audiometry multiple standardize hearing assessment despite the technical limita- methods were used for evaluating the thresholds of different tions. These efforts facilitated the development of the frequencies. Tuning forks were important for lower frequen- audiometer, and continue to influence clinical practice even cies, whisper, and speech for mid-frequencies, and Galton’s today. Key Words: American Otological Society— whistle and Konig’s rod evaluated high frequencies. Hearing Hearing assessment—History of otology. threshold was often recorded as in terms of duration of a sound, or distance from the source, rather than intensity. Hearing ability was often recorded a fraction, for example, Otol Neurotol 39:S30–S42, 2018. -
Otoscopy and Tympanometry Screening Manual
OTOSCOPY AND TYMPANOMETRY SCREENING Community & Family Health Division Maternal & Child Health Section Child & Adolescent Health Unit Revised July 2009, Reviewed April 2011 For more information contact: Minnesota Department of Health Community & Family Health Division/Maternal & Child Health Section/ Child & Adolescent Health Unit 85 East Seventh Place P.O. Box 64882-0882 St. Paul, MN 55164-0882 651-201-3760 Printed on recycled paper, 2011. Upon request, this publication can be made available in alternative formats such as large prints, Braille or cassette tape. Otoscopy and Tympanometry Workshop A comprehensive hearing-screening program consists of four major components, hearing history, visual inspection (both external and otoscopic), tympanometry and pure tone audiometry. This program will provide instruction for visual inspection, otoscopy and tympanometry only. Please refer to the MDH website (www.health.state.mn.us/divs/fh/mch) for instruction regarding hearing history and puretone audiometry. Table of Contents Page Tympanometry/Otoscopy Professional Recommendations 1 Otoscopy Visual Inspection Screening Procedures 2 External Visual Inspection 3 The Otoscope 4 Using the Otoscope Correctly 5 Tympanometry Tympanometry Screening Overview 6 Parameters of Tympanometry Screening 7-9 Tympanometer Overview 10 MDH Tympanometry Guidelines 11 Screening Protocol for Hearing and Tympanometry Screening 12 Child/Adult Referral Parameters 13 Common Tympanometry Graph 14 Middle Ear Problems and Possible Tympanometry Patterns 15 Glossary 16 Tympanometry Reading Explanation 17 Infection Control 18 Hearing Resources on the World Wide Web 19 Tympanometry and Otoscopy Professional Recommendations The following are recommendations from two major authorities for hearing assessment in children, one is the American Speech-Language Hearing Association (ASHA) and the other is the American Academy of Pediatrics (AAP).