Cellular Identity and Ca2+ Signaling Activity of the Non
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

List of Participating Universities of the HUMAP
List of Participating Universities of the HUMAP (As of April, 2015) Japan Ashiya University (Taiwan) Kai Nan University (Hyogo) Himeji Dokkyo University National Kaohsiung First University of Science and Technology (25) Hyogo University National Taichung University Hyogo University of Teacher Education National Taipei University Kansai University of International Studies National Taiwan University of Arts Kobe City College of Nursing National Taiwan Ocean University Kobe City University of Foreign Studies National Yunlin University of Science and Technology Kobe College Providence University Kobe Design University Shu-Te University Kobe Gakuin University Southern Taiwan University of Technology Kobe International University Tunghai University Kobe Pharmaceutical University Indonesia Airlangga Univeresity Kobe Shinwa Women's University (11) Bung Hatta University Kobe Shoin Women's University Darma Persada University Kobe University Gadjah Mada University Kobe Women's University Hasanuddin University Konan University Institut Teknologi Bandung Konan Women's University Institut Teknologi Sepuluh Nopember Koshien University Satya Wacana Christian University Kwansei Gakuin University Syiah Kuala University Mukogawa Women's University Udayana University Otemae University University of Indonesia Sonoda Women's University Korea Ajou University University of Hyogo* (29) Cheju National University University of Marketing and Distribution Sciences Chosun University Dong-A University Australia Australian Maritime College Dong Seo University (11) Curtin -

Japanese ACCOUNTING FORUM 2009 No. 17
Japanese ACCOUNTING FORUM 2009 No. 17 JAPAN ACCOUNTING ASSOCIATION Japan Accounting Association. Liaison Office: Hayashi Building, 1-10 Kanda Nishiki-cho, Chiyoda-ku, Tokyo 101-0054, Japan Copyright© 2009, Japan Accounting Association 1 Japanese ACCOUNTING FORUM 2009 JAPAN ACCOUNTING ASSOCIATION PREFACE Japanese ACCOUNTING FORUM of Japan Accounting Association (JAA) is published annually to publicize academic activities of JAA in English. The first issue of Japanese ACCOUNTING FORUM was published in 1993. This edition for 2009 is the 17th issue of Japanese ACCOUNTING FORUM. This issue contains the summary of presentations at the 67th Annual Conference of JAA which was held at Rikkyo University in Tokyo on September 8-10, 2008. It also includes the reports of regional activities of JAA during the 2008 academic year. I sincerely hope that Japanese ACCOUNTING FORUM serves the readers to better understand the activities of JAA. Kazuo Hiramatsu Chairman of the International Committee and Managing Editor of Japanese ACOUNTING FORUM, Japan Accounting Association Contact: Professor Kazuo Hiramatsu School of Business Administration Kwansei Gakuin University 1-1-155 Uegahara, Nishinomiya, Hyogo 662-8501, Japan [email protected] 2 JAPAN ACCOUNTING ASSOCIATION Board of Directors (2006-2009) President: Shizuki Saito, Meiji Gakuin University Directors: Hideyoshi Ando, Hitotsubashi University Tadashi Ishizaki, Chuo University Teruyuki Kawasaki, Konan University Keiko Kitamura, Chuo University Yoshinao Kozuma, Sophia University Chitoshi Koga, Kobe -

Participating HUMAP Universities
Participating HUMAP Universities Area the name of the university Area the name of the university Universities Japan Ashiya University (Taipei China) KaiNan University National Kaohsiung First University of in Hyogo (26) Himeji Dokkyo University Science and Technology (26) Hyogo University NationalTaichung University of Education Hyogo University of Teacher Education National Taipei University Kansai University of International Studies National Taiwan University of Arts Kobe City College of Nursing National Taiwan Ocean University National Yunlin University of Science Kobe City University of Foreign Studies and Technology Kobe College National United University Kobe Design University Providence University Kobe Gakuin University Shu Te University Southern Taiwan University of Science Kobe International University and Technology Kobe Pharmaceutical University Tunghai University Kobe Shinwa Women's University National Central University Kobe Shoin Women's University Indonesia Airlangga Univeresity Kobe University (11) Bung Hatta University Kobe Women's University Darma Persada University Konan University Gadjah Mada University Konan Women's University Hasanuddin University Koshien University Institut Teknologi Bandung Kwansei Gakuin University Institut Teknologi Sepuluh Nopember Mukogawa Women's University Satya Wacana Christian University Otemae University Syiah Kuala University Sonoda Women's University Udayana University University of Hyogo University of Indonesia University of Marketing and Distribution Sciences Korea Ajou University -

Graduate School Overview
AY 2019 Graduate School Overview <Reference Only> Osaka City University Table of Contents Page History ・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・ 1 Enrollment Quotas ・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・ 1 Research Fields and Classes Graduate School of Business ・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・ 2 Graduate School of Economics ・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・ 4 Graduate School of Law ・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・ 5 Graduate School of Literature and Human Sciences ・・・・・・・・・・・・・・・ 7 Graduate School of Science ・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・ 12 Graduate School of Engineering ・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・ 15 Graduate School of Medicine ・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・ 19 Graduate School of Nursing ・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・ 26 Graduate School of Human Life Science ・・・・・・・・・・・・・・・・・・・・・・・・・・・28 Graduate School for Creative Cities ・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・ 31 Graduate School of Urban Management ・・・・・・・・・・・・・・・・・・・・・・・・・・・32 Degrees ・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・34 Entrance Examinations ・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・35 Alma Maters of Enrollees ・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・ 40 Graduate School Exam Schedule (tentative) ・・・・・・・・・・・・・・・・・・・・・・・・・・・42 Directions ・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・・44 History■ History Osaka City University, the foundation of this graduate school, was established using a reform of the Japanese educational system in 1949 as an opportunity to merge the former -

Final Program
Sponsored by Institute of Systems, Control and Information Engineers (ISCIE) In Cooperated with The Society of Instrument and Control Engineers (SICE) The Institute of Electrical Engineers of Japan (IEE) The Institute of Electronics, Information and Communication Engineers (IEICE) The Japan Society for Industrial and Applied Mathematics (JSIAM) Information Processing Society of Japan (IPSJ) Research Institute of Signal Processing Japan (RISP) IEEE Control Systems Society Japan Chapter IEEE Control Systems Society Kansai Chapter IEEE Signal Processing Society Tokyo Joint Chapter IEEE Signal Processing Society Kansai Chapter and Hiroshima Institute of Technology Sessions (Rooms) Friday November 3rd. 2017 10:00-10:25 Opening (201) 10:25-10:45 Break 03A1 (201) 03B1 (301) 03C1 (402) System identification and Medical and biomedical Time series analysis and 10:45-12:00 parameter estimation systems I stochastic optimization Chair: Yoshiki Takeuchi Chair: Yukihiro Kubo Chair: Ihor Lubashevsky (Osaka Kyoiku University) (Ritsumeikan University) (Aizu University) 12:00-13:30 Lunch 03A2 (501) 03C2 (402) Modeling, filtering and 03B2 (301) Signal detection and Check-in control of stochastic Medical and biomedical statistical signal desk 13:30-14:45 systems I systems II processing Presenters' and Secretariat Chair: Kenji Sugimoto Chair: Kunihiko Oura Chair: Hiroyuki Fujioka preparation coffee (303) (Nara Institute of Science and (Kokushikan University) (Fukuoka Institute of (403) lounge Technology) Technology) (101) 09:00-18:00 14:45-15:05 Break 09:00-18:00 -

Hicells 2020)
Hawaiʻi International Conference on English Language and Literature Studies (HICELLS 2020) “Trends in Research and Pedagogical Innovations in English Language and Literature” CONFERENCE PROGRAM March 13 -14, 2020 HICELLS 2020 03/13-14/2020 Hawaii International Conference on English Language and Literature Studies (HICELLS 2020) English Department University of Hawaii at Hilo March 13-14, 2020 CONFERENCE PROGRAM DAY 1 (FRIDAY) March 13, 2020 Time Venue: UCB 100 8:00 – 8:30 Conference Registration 8:30 – 8:45 Kipaepae 8:45 – 9:00 Welcome Address Dr Bonnie D. Irwin Chancellor University of Hawaii at Hilo 9:00 – 9:45 Keynote Address 1 Minds, Machines and Language: What Does the Future Hold? William O’Grady University of Hawaii at Manoa Hawaii, USA BREAK: UCB 127 9:45- 10:00 PARALLEL SESSION 1 10:00 – 12:20 Room: UCB 127 (10:00-12:20) No. Presenters Papers 1 Michio Hosaka The Emergence of Functional Projections in the Nihon University History of English 2 Susana T. Udoka A Study of English and Annang Clause Syntax: Akwa Ibom State University From the View Point of Grammaticality and Global Obio Akpa Campus Intelligibility HICELLS 2020 2 HICELLS 2020 03/13-14/2020 3 Quentin C. Sedlacek Contestation, Reification, and African American California State University English in College Linguistics Courses Monterey Bay 4 Hiromi Otaka On the Aspect Used in the Subordinate Clause of Kwansei Gakuin University “This is the first time ~” in English 5 Chiu-ching Tseng English Word Boundary Perception by Mandarin George Mason University Native Speakers 6 Yumiko Mizusawa An Analysis of Lexicogrammatical and Semantic Seijo University Features in Academic Writing by Japanese EFL Learners 7 Noriko Yoshimura Japanese EFL Learners’ Structural University of Shizuoka Misunderstanding: ECM Passives in L2 English Mineharu Nakayama The Ohio State University Atsushi Fujimori University of Shizuoka Room: UCB 101 (10:00-12:20) No. -

“History for Democracy in the Age of Populism”
NHCM International Conference 2019 “History for Democracy in the Age of Populism” 31 August - 1 September 2019 Kwansei Gakuin Hall, Kwansei Gakuin University Korean-Japanese Forum of Western History Interdisciplinary Research Project on the Function of National Histories and Collective Memories for the Democracy in the Globalized Society Program Program31 August 2019 10:30 Opening Remarks Nobuya Hashimoto (Kwansei Gakuin University, Japan) 10:45 Keynote I Engaging Populisms: Which Historical Memory for What Kind of Democracy? Stefan Berger (Ruhr University Bochum, Germany) 12:00 Lunch 13:30 Session I Politics of Identity, Belonging and Exclusion in the Age of Populism Takumi Itabashi (Seikei University, Japan) “Populism and -or versus- Democracy: The Experience of Modern German History” Eva-Clarita Pettai (University of Jena, Germany) “Retrospective Justice and Identity Politics in Central and Eastern Europe” Jung Han Kim (Sogang University, South Korea) “Is Populism the exit of a Democratic Crisis? : Debates on Left Populism in Korea” Commentator: Ariyoshi Ogawa (Rikkyo University, Japan) Chairperson: Hiroshi Fukuda (Seijo University, Japan) 16:00 Coffee Break 16:15 Keynote II Beyond Multidirectional Memory: Tracing the Holocaust in the African Diaspora Eve Rosenhaft (University of Liverpool, UK/ Sogang University, South Korea) 18:00 Reception 1 September 2019 10:00 Session II Revised Histories and Memories under Democratization and Transitional Justice, and their Outcome Eugenia Gay (National University of Cordoba, Argentina) “To Rebuild -

KONAN UNIVERSITY International Student Workshop 2021
KONAN UNIVERSITY International Student Workshop 2021 Konan University warmly welcomes all students navigating the path to a new normal to join an innovative three-part online culture and language exchange student workshop. Participants will explore critical elements of global citizenship and develop intercultural competence in the process. Reaching across physical borders, participants have a platform to voice their thoughts and share their perspectives and experiences in an international collaborative effort towards a sustainable global society. Theme: Exploring Global Citizenship During and After Covid-19 The novel coronavirus has disrupted the lives of people across all parts of the world, significantly affecting the most vulnerable and their families and communities. Access to educational opportunities has decreased, and many students find it necessary at best to adapt to changes and limitations and at worse to put aside their aspirations. For students seeking to participate in overseas study abroad programs, their pursuits have been interrupted due to restrictions in physical mobility across borders. Until these restrictions are lifted, and physical mobility is no longer limited, efforts to promote the mobility of students’ lived experience and ideas are vital to broadening perspectives that sustain global citizenship education for students across the world. Objective: To provide opportunities to students that promote culture and language exchange in English and Japanese, bring awareness to issues that people face in countries at the local level, develop intercultural competence, and foster global citizenship through meaningful student exchange. Method: The three-part workshop will be conducted using a combination of virtual Zoom meetings and educational tools including Flipgrid and Padlet. -
Tentative Workshop Program
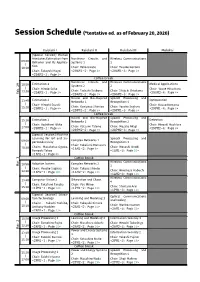
Session Schedule (*tentative ed. as of February 20, 2020) Kaiulani I Kaiulani II Kaiulani III Molokai [Special Session] Human Attributes Estimation from Nonlinear Circuits and Wireless Communications 09:00 Behavior and Its Applica- Systems 1 1 | tion 10:30 Chair: Yoko Uwate Chair: Yusuke Kozawa Chair: Takayuki Nagai <29AM1-2: Page 1> <29AM1-3: Page 2> <29AM1-1: Page 1> Coffee break Nonlinear Circuits and Wireless Communications Estimation 1 Medical Applications 10:50 Systems 2 2 | Chair: Hiroto Saito Chair: Yasue Mitsukura 12:20 Chair: Tadashi Tsubone Chair: Shigeki Shiokawa Feb. 29 <29AM2-1: Page 2> <29AM2-4: Page 4> <29AM2-2: Page 3> <29AM2-3: Page 3> Neural and Bio-Inspired Speech Processing and Estimation 2 Optimization 13:40 Networks 1 Recognition 1 | Chair: Hiroshi Suzuki Chair: Kazuo Komatsu Chair: Kuniyasu Shimizu Chair: Yosuke Sugiura 15:10 <29PM1-1: Page 4> <29PM1-4: Page 6> <29PM1-2: Page 5> <29PM1-3: Page 5> Coffee break Neural and Bio-Inspired Speech Processing and Estimation 3 Detection 15:30 Networks 2 Recognition 2 | Chair: Yoshifumi Ukita Chair: Hiroaki Hashiura Chair: Katsumi Tateno Chair: Masato Akagi 17:00 <29PM2-1: Page 6> <29PM2-4: Page 8> <29PM2-2: Page 7> <29PM2-3: Page 7> [Special Session] Machine Learning for IoT and Su- Speech Processing and Complex Networks 1 09:00 perconductivity Recognition 3 | Chair: Takafumi Matsuura Chairs: Masakatsu Ogawa, Chair: Masashi Unoki 10:30 <1AM1-2: Page 9> Tomoaki Takao <1AM1-3: Page 10> <1AM1-1: Page 8> Coffee break Wireless Communications 10:50 Adaptive System Complex Networks -

Study Tour of “The Kobe Shimbun” Newspaper Attention International Students Staying in Kobe!
The Project for Promoting an International Exchange at the Hyogo International House Great Chance to Explore One of Kobe’s Local Industries! Study Tour of “The Kobe Shimbun” Newspaper Attention International Students Staying in Kobe! Soon to celebrate the 120th anniversary of its foundation in February 2018, the local Japanese-language newspaper Kobe Shimbun is one of the leading companies of Kobe and its surrounding areas. The paper is not only engaged in simply disseminating news and information. It also has linkages with institutions such as Kobe University, Kwansei Gakuin University and Konan University. Kobe Shimbun deploys “Knowledge Power” derived from such arrangements toward realizing a sound and healthy “Land”, in giving a helping hand in boosting the local economy as well as promoting disaster prevention and mitigation, in addition to generating an environment for safe child-rearing. Based on its information-transmitting as well as networking power unique to a local newspaper, Kobe Shimbun is committed to promoting people-to-people connection and a further widening of such circles, thereby helping to shape the local society and its future better than ever before. We invite you to gain a closer knowledge of a part (editorial) of the newspaper-making process starting with editorial work up through printing and delivery. At the same time, you would learn about how the paper reports on disasters and disaster prevention, through which we hope you would come to feel a greater closeness to Kobe Shimbun and deepen your understanding of its work. You are all very welcome to take part in this very informative and interesting study tour! Date & Time : Jan. -

1. Japanese National, Public Or Private Universities
1. Japanese National, Public or Private Universities National Universities Hokkaido University Hokkaido University of Education Muroran Institute of Technology Otaru University of Commerce Obihiro University of Agriculture and Veterinary Medicine Kitami Institute of Technology Hirosaki University Iwate University Tohoku University Miyagi University of Education Akita University Yamagata University Fukushima University Ibaraki University Utsunomiya University Gunma University Saitama University Chiba University The University of Tokyo Tokyo Medical and Dental University Tokyo University of Foreign Studies Tokyo Geijutsu Daigaku (Tokyo University of the Arts) Tokyo Institute of Technology Tokyo University of Marine Science and Technology Ochanomizu University Tokyo Gakugei University Tokyo University of Agriculture and Technology The University of Electro-Communications Hitotsubashi University Yokohama National University Niigata University University of Toyama Kanazawa University University of Fukui University of Yamanashi Shinshu University Gifu University Shizuoka University Nagoya University Nagoya Institute of Technology Aichi University of Education Mie University Shiga University Kyoto University Kyoto University of Education Kyoto Institute of Technology Osaka University Osaka Kyoiku University Kobe University Nara University of Education Nara Women's University Wakayama University Tottori University Shimane University Okayama University Hiroshima University Yamaguchi University The University of Tokushima Kagawa University Ehime -

Name of Who Letter Is Addressed
Curriculum Vitae Center for Pediatric Auditory and Speech Sciences Nemours Biomedical Research Kyoko Nagao, Ph.D. 1701 Rockland Road, Wilmington, DE 19803 URL: www.asel.udel.edu/~nagao/ Last updated on May, 2017 Phone: 302-651-6830 Email: [email protected] Professional Experience 2010-Present Nemours / Alfred I. duPont Hospital for Children Center for Pediatric Auditory & Speech Sciences Assistant Research Scientist, Auditory Physiology & Psychoacoustics Research Laboratory • Investigating the auditory efferent and afferent functioning in children with Auditory Processing Disorders • Development of aural (re)habilitation software for infants and toddlers with severe to profound hearing loss, including children with cochlear implants • Investigating effects of medication on auditory function in children with ADHD • Supervising undergraduate and graduate student assistants 2007-2010 Nemours / Alfred I. duPont Hospital for Children Center for Pediatric Auditory & Speech Sciences Postdoctoral Fellow, Speech Research Laboratory • Development and evaluation of a text-to-speech synthesis program • Longitudinal study of speech development in young children with cochlear implants 2008-Present University of Delaware, Department of Linguistics and Cognitive Science Adjunct Assistant Professor 2006-2007 Indiana University, Department of Speech & Hearing Sciences Postdoctoral Researcher, Voice Physiology Laboratory 2000-2006 Indiana University, Department of Linguistics Graduate Research Assistant 2000 NTT Basic Research Laboratories, Atsugi,