1,6-Dehydropinidine Is an Abundant Compound in Picea Abies
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Two New Chrysomyxa Rust Species on the Endemic Plant, Picea Asperata in Western China, and Expanded Description of C
Phytotaxa 292 (3): 218–230 ISSN 1179-3155 (print edition) http://www.mapress.com/j/pt/ PHYTOTAXA Copyright © 2017 Magnolia Press Article ISSN 1179-3163 (online edition) https://doi.org/10.11646/phytotaxa.292.3.2 Two new Chrysomyxa rust species on the endemic plant, Picea asperata in western China, and expanded description of C. succinea JING CAO1, CHENG-MING TIAN1, YING-MEI LIANG2 & CHONG-JUAN YOU1* 1The Key Laboratory for Silviculture and Conservation of Ministry of Education, Beijing Forestry University, Beijing 100083, China 2Museum of Beijing Forestry University, Beijing 100083, China *Corresponding author: [email protected] Abstract Two new rust species, Chrysomyxa diebuensis and C. zhuoniensis, on Picea asperata are recognized by morphological characters and DNA sequence data. A detailed description, illustrations, and discussion concerning morphologically similar and phylogenetically closely related species are provided for each species. From light and scanning electron microscopy observations C. diebuensis is characterized by the nailhead to peltate aeciospores, with separated stilt-like base. C. zhuoni- ensis differs from other known Chrysomyxa species in the annulate aeciospores with distinct longitudinal smooth cap at ends of spores, as well as with a broken, fissured edge. Analysis based on internal transcribed spacer region (ITS) partial gene sequences reveals that the two species cluster as a highly supported group in the phylogenetic trees. Correlations between the morphological and phylogenetic features are discussed. Illustrations and a detailed description are also provided for the aecia of C. succinea in China for the first time. Keywords: aeciospores, molecular phylogeny, spruce needle rust, taxonomy Introduction Picea asperata Mast.is native to western China, widely distributed in Qinghai, Gansu, Shaanxi and western Sichuan. -

Clarification of the Life-Cycle of Chrysomyxa Woroninii on Ledum
Mycol. Res. 104 (5): 581–586 (May 2000). Printed in the United Kingdom. 581 Clarification of the life-cycle of Chrysomyxa woroninii on Ledum and Picea Patricia E. CRANE1, 2, Yasuyuki HIRATSUKA2 and Randolph S. CURRAH1 " Department of Biological Sciences, University of Alberta, Edmonton, AB T6G 2E9, Canada # Northern Forestry Centre, Canadian Forest Service, 5320-122 Street, Edmonton, AB T6H 3S5, Canada. Accepted 5 August 1999. The rust fungus Chrysomyxa woroninii causes perennial witches’ brooms on several species of Ledum in northern and subalpine regions of Europe, North America and Asia. Spruce bud rust has been assumed to be the aecial state of C. woroninii because of the close proximity of infected Ledum plants and systemically infected buds on Picea. The lack of experimental evidence for this connection, however, and the presence of other species of Chrysomyxa on the same hosts has led to confusion about the life-cycle of C. woroninii. In this study, infections on both spruce and Ledum were studied in the field and in a greenhouse. The link between the two states was proven by inoculating spruce with basidiospores from Ledum groenlandicum. After infection of spruce in spring, probably through the needles, the fungus overwinters in the unopened buds until the next spring, when the infected shoots are distinguished by stunting and yellow or red discolouration. Microscopic examination of dormant Ledum shoots showed that C. woroninii overwinters in this host in the bracts and outer leaves of the vegetative buds, and in the pith and cortex of the stem. The telia of C. woroninii, on systemically infected Ledum leaves of the current season, are easily distinguished from the telia of other Chrysomyxa species on the same hosts. -

The Anatomy of Spruce Needles '
THE ANATOMY OF SPRUCE NEEDLES ' By HERBERT F. MARCO 2 Junior forester. Northeastern Forest Experiment Station,^ Forest Service, United States Department of Agriculture INTRODUCTION In 1865 Thomas (16) * made a comparative study of the anatomy of conifer leaves and fomid that the structural variations exhibited by the different species warranted taxonomic considération. Since that time leaf anatomy has become a fertile and interesting field of research. Nearly all genera of gymnosperms have received some attention, and the literature on this subject has become voluminous. A detailed review of the literature will not be attempted in this paper, since com- prehensive reviews have already been published by Fulling (6) and Lacassagne (11). FuUing's paper contains in addition an extensive bibliography on conifer leaf anatomy. Most workers in tMs field of research have confined their efforts to the study of the cross sections of needles. This is partly because longitudinal sections are difficult to obtain and partly because they present but little structural variation of value for identification. The workers who have studied both longitudinal and cross sections have restricted their descrii)tions of longitudinal sections either to specific tissues or to a few species of a large number of genera, and the descrip- tions, although comprehensive, leave much to be desired from the standpoint of detailed information and illustration. Domer (S) was perhaps the first to use sketches to augment keys to and descriptions of the native firs and spruces. His diagrammatic sketches portray the shape of the needles in cross section and the position of the resin canals. Durrell (4) went a step further and illustrated his notes on the North American conifers by camera-lucida drawing^ depicting the orientation and arrangement of the various needle tissues in cross section. -

9B Taxonomy to Genus
Fungus and Lichen Genera in the NEMF Database Taxonomic hierarchy: phyllum > class (-etes) > order (-ales) > family (-ceae) > genus. Total number of genera in the database: 526 Anamorphic fungi (see p. 4), which are disseminated by propagules not formed from cells where meiosis has occurred, are presently not grouped by class, order, etc. Most propagules can be referred to as "conidia," but some are derived from unspecialized vegetative mycelium. A significant number are correlated with fungal states that produce spores derived from cells where meiosis has, or is assumed to have, occurred. These are, where known, members of the ascomycetes or basidiomycetes. However, in many cases, they are still undescribed, unrecognized or poorly known. (Explanation paraphrased from "Dictionary of the Fungi, 9th Edition.") Principal authority for this taxonomy is the Dictionary of the Fungi and its online database, www.indexfungorum.org. For lichens, see Lecanoromycetes on p. 3. Basidiomycota Aegerita Poria Macrolepiota Grandinia Poronidulus Melanophyllum Agaricomycetes Hyphoderma Postia Amanitaceae Cantharellales Meripilaceae Pycnoporellus Amanita Cantharellaceae Abortiporus Skeletocutis Bolbitiaceae Cantharellus Antrodia Trichaptum Agrocybe Craterellus Grifola Tyromyces Bolbitius Clavulinaceae Meripilus Sistotremataceae Conocybe Clavulina Physisporinus Trechispora Hebeloma Hydnaceae Meruliaceae Sparassidaceae Panaeolina Hydnum Climacodon Sparassis Clavariaceae Polyporales Gloeoporus Steccherinaceae Clavaria Albatrellaceae Hyphodermopsis Antrodiella -

BDB 2014 Picea Study Day, an Introduction
BDB 2014 Picea study day, an introduction, by Paul Goetghebeur, BG Ghent University Scan Jan De Langhe : Picea_asperata_(not_heterolepis)_NPVBM19842407_2950JDL_15022007_10.JPG From ferns to Gymnosperms : from sporangia to seeds Seed ferns : Medullosaceae (fossil) (Kalkman 1972) A. Medullosa noei, habit B. Id., stele in cross section C. Medullosa solmsii, id. D. Medullosa luckartii, id. E. Alethopteris lancifolia, pinna F. Neuropteris, pinna with seed G. Trigonocarpus, ovule longitudinal section H. Id., cross section Seed ferns (fossil) (Stewart 1983) 1. Archaeosperma arnoldii, ovules The integument is almost entirely united with the nucellus (except for the top). In the nucellus a large macroprothallium (= female gametophyte) is evident. 2. Reconstruction of a semophylesis explaining the origin of the ovule. A : basic pattern with dichotomous branching and terminal sporangia B : start of heterospory, with one macrosporangium surrounded by many microsporangia C : microsporangia are reduced, their telomes are “sterile” D : webbing of the “sterile” telomes around the macrosporangium resulting in the formation of a new layer, this new layer = the integument (later on forming the seed coat !) Development of the ovule in Gymnosperms (Kalkman 1972) A : full grown ovule B : primordium of ovule, with a small nucellus, surrounded by an incipient integument C : differentiation of the macrospore mother cell (still diploid !) in the nucellus (= macrosporangium wall) D : meiosis of the macrospore mother cell, yielding 4 haploid macrospores E : degeneration -

A New Rust Species of Diaphanopellis on Rhododendron Oreodoxa from Southern China
Phytotaxa 309 (1): 055–065 ISSN 1179-3155 (print edition) http://www.mapress.com/j/pt/ PHYTOTAXA Copyright © 2017 Magnolia Press Article ISSN 1179-3163 (online edition) https://doi.org/10.11646/phytotaxa.309.1.5 A new rust species of Diaphanopellis on Rhododendron oreodoxa from Southern China JING CAO1, CHENG-MING TIAN1, YING-MEI LIANG2 & CHONG-JUAN YOU1* 1The Key Laboratory for Silviculture and Conservation of Ministry of Education, Beijing Forestry University, Beijing 100083, China 2Museum of Beijing Forestry University, Beijing 100083, China *Corresponding author: [email protected] Abstract A novel rust species Diaphanopellis purpurea on Rhododendron oreodoxa collected in Southern China was identified and described. Light and scanning electron microscopy observations indicated that this rust species was morphologically distinct from other known Diaphanopellis species and Chrysomyxa species in teliospore morphology and urediniospore surface structure. Diaphanopellis purpurea can be phylogenetically separated from other Chrysomyxa species based on analysis of internal transcribed spacer (ITS) partial gene sequences. The aecial stage of the new species was also confirmed. Keywords: Molecular phylogeny, phylogeny, Pucciniales, taxonomy Introduction Rust genus Diaphanopellis was established by Crane with the type species Diaphanopellis forrestii P. E. Crane occurring on Rhododendron selense Franch (Crane 2005, Kirk et al. 2008). Diaphanopellis is characterized by the teliospores enclosed in hyaline sheaths, and the uredinia surrounded by a peridium with ornamented cells (Barclay 1891, Balfour-Browne 1955, Crane 2005). Most rusts infecting Rhododendron belong to the genus Chrysomyxa, which are morphologically different from Diaphanopellis species in having uredinial peridium and distinct teliospores. Chrysomyxa species produce catenulate teliospores without gelatineous layers and uredinia covered by an inconspicuous peridium (Berndt 1999). -

Sites of Importance for Nature Conservation Wales Guidance (Pdf)
Wildlife Sites Guidance Wales A Guide to Develop Local Wildlife Systems in Wales Wildlife Sites Guidance Wales A Guide to Develop Local Wildlife Systems in Wales Foreword The Welsh Assembly Government’s Environment Strategy for Wales, published in May 2006, pays tribute to the intrinsic value of biodiversity – ‘the variety of life on earth’. The Strategy acknowledges the role biodiversity plays, not only in many natural processes, but also in the direct and indirect economic, social, aesthetic, cultural and spiritual benefits that we derive from it. The Strategy also acknowledges that pressures brought about by our own actions and by other factors, such as climate change, have resulted in damage to the biodiversity of Wales and calls for a halt to this loss and for the implementation of measures to bring about a recovery. Local Wildlife Sites provide essential support between and around our internationally and nationally designated nature sites and thus aid our efforts to build a more resilient network for nature in Wales. The Wildlife Sites Guidance derives from the shared knowledge and experience of people and organisations throughout Wales and beyond and provides a common point of reference for the most effective selection of Local Wildlife Sites. I am grateful to the Wales Biodiversity Partnership for developing the Wildlife Sites Guidance. The contribution and co-operation of organisations and individuals across Wales are vital to achieving our biodiversity targets. I hope that you will find the Wildlife Sites Guidance a useful tool in the battle against biodiversity loss and that you will ensure that it is used to its full potential in order to derive maximum benefit for the vitally important and valuable nature in Wales. -

Notes, Outline and Divergence Times of Basidiomycota
Fungal Diversity (2019) 99:105–367 https://doi.org/10.1007/s13225-019-00435-4 (0123456789().,-volV)(0123456789().,- volV) Notes, outline and divergence times of Basidiomycota 1,2,3 1,4 3 5 5 Mao-Qiang He • Rui-Lin Zhao • Kevin D. Hyde • Dominik Begerow • Martin Kemler • 6 7 8,9 10 11 Andrey Yurkov • Eric H. C. McKenzie • Olivier Raspe´ • Makoto Kakishima • Santiago Sa´nchez-Ramı´rez • 12 13 14 15 16 Else C. Vellinga • Roy Halling • Viktor Papp • Ivan V. Zmitrovich • Bart Buyck • 8,9 3 17 18 1 Damien Ertz • Nalin N. Wijayawardene • Bao-Kai Cui • Nathan Schoutteten • Xin-Zhan Liu • 19 1 1,3 1 1 1 Tai-Hui Li • Yi-Jian Yao • Xin-Yu Zhu • An-Qi Liu • Guo-Jie Li • Ming-Zhe Zhang • 1 1 20 21,22 23 Zhi-Lin Ling • Bin Cao • Vladimı´r Antonı´n • Teun Boekhout • Bianca Denise Barbosa da Silva • 18 24 25 26 27 Eske De Crop • Cony Decock • Ba´lint Dima • Arun Kumar Dutta • Jack W. Fell • 28 29 30 31 Jo´ zsef Geml • Masoomeh Ghobad-Nejhad • Admir J. Giachini • Tatiana B. Gibertoni • 32 33,34 17 35 Sergio P. Gorjo´ n • Danny Haelewaters • Shuang-Hui He • Brendan P. Hodkinson • 36 37 38 39 40,41 Egon Horak • Tamotsu Hoshino • Alfredo Justo • Young Woon Lim • Nelson Menolli Jr. • 42 43,44 45 46 47 Armin Mesˇic´ • Jean-Marc Moncalvo • Gregory M. Mueller • La´szlo´ G. Nagy • R. Henrik Nilsson • 48 48 49 2 Machiel Noordeloos • Jorinde Nuytinck • Takamichi Orihara • Cheewangkoon Ratchadawan • 50,51 52 53 Mario Rajchenberg • Alexandre G. -

Host Jumps Shaped the Diversity of Extant Rust Fungi (Pucciniales)
Research Host jumps shaped the diversity of extant rust fungi (Pucciniales) Alistair R. McTaggart1, Roger G. Shivas2, Magriet A. van der Nest3, Jolanda Roux4, Brenda D. Wingfield3 and Michael J. Wingfield1 1Department of Microbiology and Plant Pathology, Tree Protection Co-operative Programme (TPCP), Forestry and Agricultural Biotechnology Institute (FABI), University of Pretoria, Private Bag X20, Pretoria 0028, South Africa; 2Department of Agriculture and Forestry, Queensland Plant Pathology Herbarium, GPO Box 267, Brisbane, Qld 4001, Australia; 3Department of Genetics, Forestry and Agricultural Biotechnology Institute (FABI), University of Pretoria, Private bag X20, Pretoria 0028, South Africa; 4Department of Plant Sciences, Tree Protection Co-operative Programme (TPCP), Forestry and Agricultural Biotechnology Institute (FABI), University of Pretoria, Private Bag X20, Pretoria 0028, South Africa Summary Author for correspondence: The aim of this study was to determine the evolutionary time line for rust fungi and date Alistair R. McTaggart key speciation events using a molecular clock. Evidence is provided that supports a contempo- Tel: +2712 420 6714 rary view for a recent origin of rust fungi, with a common ancestor on a flowering plant. Email: [email protected] Divergence times for > 20 genera of rust fungi were studied with Bayesian evolutionary Received: 8 July 2015 analyses. A relaxed molecular clock was applied to ribosomal and mitochondrial genes, cali- Accepted: 26 August 2015 brated against estimated divergence times for the hosts of rust fungi, such as Acacia (Fabaceae), angiosperms and the cupressophytes. New Phytologist (2016) 209: 1149–1158 Results showed that rust fungi shared a most recent common ancestor with a mean age doi: 10.1111/nph.13686 between 113 and 115 million yr. -

Vascular Plants of the Russian Peak Area Siskiyou County, California James P
Humboldt State University Digital Commons @ Humboldt State University Botanical Studies Open Educational Resources and Data 2-2004 Vascular Plants of the Russian Peak Area Siskiyou County, California James P. Smith Jr Humboldt State University, [email protected] Follow this and additional works at: http://digitalcommons.humboldt.edu/botany_jps Part of the Botany Commons Recommended Citation Smith, James P. Jr, "Vascular Plants of the Russian Peak Area Siskiyou County, California" (2004). Botanical Studies. 34. http://digitalcommons.humboldt.edu/botany_jps/34 This Flora of Northwest California: Checklists of Local Sites of Botanical Interest is brought to you for free and open access by the Open Educational Resources and Data at Digital Commons @ Humboldt State University. It has been accepted for inclusion in Botanical Studies by an authorized administrator of Digital Commons @ Humboldt State University. For more information, please contact [email protected]. VASCULAR PLANTS OF THE RUSSIAN PEAK AREA SISKIYOU COUNTY, CALIFORNIA Edited by John O. Sawyer, Jr. & James P. Smith, Jr. Professor Emeritus of Botany Department of Biological Sciences Humboldt State University Arcata, California 18 February 2004 Russian Peak (elevation 8196 ft.) is located in the Salmon Mountains, about 12.5 miles south-southwest FLOWERING PLANTS of Etna. It is the highest peak in the Russian Wilderness. The Salmon Mountains are a subunit of Aceraceae the Klamath Mountains. The area is famous for its Acer glabrum var. torreyi diversity of conifer species and for the discovery of the subalpine fir in California, based on the field work Apocynaceae of John Sawyer and Dale Thornburgh. Apocynum androsaemifolium FERNS Berberidaceae Mahonia dictyota Equisetaceae Mahonia nervosa var. -
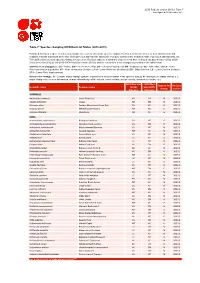
Table 7: Species Changing IUCN Red List Status (2012-2013)
IUCN Red List version 2013.2: Table 7 Last Updated: 25 November 2013 Table 7: Species changing IUCN Red List Status (2012-2013) Published listings of a species' status may change for a variety of reasons (genuine improvement or deterioration in status; new information being available that was not known at the time of the previous assessment; taxonomic changes; corrections to mistakes made in previous assessments, etc. To help Red List users interpret the changes between the Red List updates, a summary of species that have changed category between 2012 (IUCN Red List version 2012.2) and 2013 (IUCN Red List version 2013.2) and the reasons for these changes is provided in the table below. IUCN Red List Categories: EX - Extinct, EW - Extinct in the Wild, CR - Critically Endangered, EN - Endangered, VU - Vulnerable, LR/cd - Lower Risk/conservation dependent, NT - Near Threatened (includes LR/nt - Lower Risk/near threatened), DD - Data Deficient, LC - Least Concern (includes LR/lc - Lower Risk, least concern). Reasons for change: G - Genuine status change (genuine improvement or deterioration in the species' status); N - Non-genuine status change (i.e., status changes due to new information, improved knowledge of the criteria, incorrect data used previously, taxonomic revision, etc.) IUCN Red List IUCN Red Reason for Red List Scientific name Common name (2012) List (2013) change version Category Category MAMMALS Nycticebus javanicus Javan Slow Loris EN CR N 2013.2 Okapia johnstoni Okapi NT EN N 2013.2 Pteropus niger Greater Mascarene Flying -

Life at the Margin: the Mating System of Mediterranean Conifers
Web Ecology 8: 94–102. Life at the margin: the mating system of Mediterranean conifers Gwendal Restoux, Daniel E. Silva, Fabrice Sagnard, Franck Torre, Etienne Klein and Bruno Fady Restoux, G., Silva, D. E., Sagnard, F., Torre, F., Klein, E. and Fady, B. 2008. Life at the margin: the mating system of Mediterranean conifers. – Web Ecol. 8: 94–102. Mixed mating, where a single tree progeny results from a mixture of selfing and out- crossing, is widespread in conifers and could be an evolutionary advantage at ecological margins when mating partners become scarce. This study analyzes how the mating system responds to bioclimate and density variations. We surveyed published data on the mating system of Abies, Picea and Pinus species when information on bioclimate and stand density was available. Our survey revealed that Mediterranean species dem- onstrate a lower selfing rate than other species and that the proportion of selfed versus outcrossed progeny is not fixed within species. The highest variability in mating types within populations was found when stand density was the most variable. To show how density affects the proportion of selfed versus outcrossed progeny, we used isozymes to genotype single tree seeds from a marginal Abies alba forest in Medi- terranean France (Mont Ventoux) where low-to high-density stands are found. We then tested the adaptive potential of the different high and low density progenies by sowing them under controlled nursery conditions and measuring germination rate and seedling survival after 4 years under 3 different water regimes. Although the mean value of outcrossing rate was typical for mixed mating conifers (tm = 0.85), individual outcrossing rates varied from 0.05 to 0.99 and were strongly correlated with stand type and density (tm from 0.87 in high-density to 0.43 in low-density marginal stands).