TECHNISCHE UNIVERSITÄT MÜNCHEN the Effect Of
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Surnames 198
Surnames 198 P PACQUIN PAGONE PALCISCO PACUCH PAHACH PALEK PAAHANA PACY PAHEL PALENIK PAAR PADASAK PAHUSZKI PALERMO PAASSARELLI PADDOCK PAHUTSKY PALESCH PABALAN PADELL PAINE PALGUTA PABLIK PADGETT PAINTER PALI PABRAZINSKY PADLO PAIRSON PALILLA PABST PADUNCIC PAISELL PALINA PACCONI PAESANI PAJAK PALINO PACE PAESANO PAJEWSKI PALINSKI PACEK PAFFRATH PAKALA PALKO PACELLI PAGANI PAKOS PALL PACEY PAGANO PALACE PALLO PACHARKA PAGDEN PALADINO PALLONE PACIFIC PAGE PALAGGO PALLOSKY PACILLA PAGLARINI PALAIC PALLOTTINI PACINI PAGLIARINI PALANIK PALLOZZI PACK PAGLIARNI PALANKEY PALM PACKARD PAGLIARO PALANKI PALMA PACKER PAGLIARULO PALAZZONE PALMER PACNUCCI PAGLIASOTTI PALCHESKO PALMERO PACOLT PAGO PALCIC PALMERRI Historical & Genealogical Society of Indiana County 1/21/2013 Surnames 199 PALMIERI PANCIERRA PAOLO PARDUS PALMISANO PANCOAST PAONE PARE PALMISCIANO PANCZAK PAPAKIE PARENTE PALMISCNO PANDAL PAPCIAK PARENTI PALMO PANDULLO PAPE PARETTI PALOMBO PANE PAPIK PARETTO PALONE PANGALLO PAPOVICH PARFITT PALSGROVE PANGBURN PAPPAL PARHAM PALUCH PANGONIS PAPSON PARILLO PALUCHAK PANIALE PAPUGA PARIS PALUDA PANKOVICH PAPURELLO PARISE PALUGA PANKRATZ PARADA PARISEY PALUGNACK PANNACHIA PARANA PARISH PALUMBO PANNEBAKER PARANIC PARISI PALUS PANONE PARAPOT PARISO PALUSKA PANOSKY PARATTO PARIZACK PALYA PANTALL PARCELL PARK PAMPE PANTALONE PARCHINSKY PARKE PANAIA PANTANI PARCHUKE PARKER PANASCI PANTANO PARDEE PARKES PANASKI PANTZER PARDINI PARKHILL PANCHICK PANZY PARDO PARKHURST PANCHIK PAOLINELLIE PARDOE PARKIN Historical & Genealogical Society of Indiana County -

Audit Final Pn 5-28-04
Appendix Radio Radio Callsign Service Licensee State Callsign Service Licensee State KA26590 IG MDOI INC TX KA96512 IG PM REALTY GROUP TX KA2774 PW OXFORD, VILLAGE OF MI KAA245 IG YELLOW & CITY CAB CO KS KA3917 IG SCRANTON TIMES PA KAD598 PW RED OAK VETERINARY CLINIC IA KA40009 IG GADSDEN, CITY OF AL KAE933 IG FOODSERVICE MANAGEMENT GROUPFL INC KA40058 IG HOUMANN, JIM:HOUMANN, CHETND KAG551 PW COOK, RICHARD L MO KA42246 IG HOUSTON FLEA MARKET INC TX KAH411 IG MIKE HOPKINS DIST CO INC TX KA42563 IG MUIRFIELD VILLAGE GOLF CLUBOH KAH535 PW CEDAR RAPIDS, CITY OF IA KA4305 IG CITY OF LOS ANGELES DEPARTMENTCA OF KAJ418WATER & POWERIG KOPSA, LEO E IA KA43600 IG SHAPLEY, CHARLES P MO KAM394 IG CROOKSTON IMPLEMENT CO INCMN KA48204 PW PRESQUE ISLE, COUNTY OF MI KAM826 IG AIRGAS SOUTHWEST INC TX KA52811 IG R & R INDUSTRIES INC MA KAM951 IG TERRA INTERNATIONAL INC IA KA53323 IG ELK RIDGE LOG INC WA KAM983 IG RAY KREBSBACH & SONS IA KA53447 PW PIERCE, TOWNSHIP OF OH KAN247 IG BROCE CONSTRUCTION CO INCKS KA53918 IG B M I INC MI KAN892 PW HIAWATHA, CITY OF KS KA61058 IG THISTLE, RONALD F MA KAO274 IG MALINE, THOMAS G NE KA62473 PW KENTUCKY, COMMONWEALTH OFKY DBA KYKAP406 EMERGENCY MANAGEMENTIG DYNEGY IT INC TX KA64283 IG SAINT MARY MEDICAL CENTERWA KAP554 IG AWARE OPERATING SERVICES TXINC KA64769 IG SOUTHERN WAREHOUSING & DISTRIBUTIONFL KAQ533 LTD PW CALIFORNIA, STATE OF CA KA65089 IG DUN & BRADSTREET NJ KAQ708 PW PENNSYLVANIA, COMMONWEALTHPA OF KA65696 IG PARSONS INFRASTRUCTURE &CA TECHNOLOGYKAR785 GROUP PW PIMA, COUNTY OF AZ KA66353 IG BALTIMORE MARINE -

Annotated Checklist of the Vascular Plants of the Washington - Baltimore Area
Annotated Checklist of the Vascular Plants of the Washington - Baltimore Area Part II Monocotyledons Stanwyn G. Shetler Sylvia Stone Orli Botany Section, Department of Systematic Biology National Museum of Natural History Smithsonian Institution, Washington, DC 20560-0166 MAP OF THE CHECKLIST AREA Annotated Checklist of the Vascular Plants of the Washington - Baltimore Area Part II Monocotyledons by Stanwyn G. Shetler and Sylvia Stone Orli Department of Systematic Biology Botany Section National Museum of Natural History 2002 Botany Section, Department of Systematic Biology National Museum of Natural History Smithsonian Institution, Washington, DC 20560-0166 Cover illustration of Canada or nodding wild rye (Elymus canadensis L.) from Manual of the Grasses of the United States by A. S. Hitchcock, revised by Agnes Chase (1951). iii PREFACE The first part of our Annotated Checklist, covering the 2001 species of Ferns, Fern Allies, Gymnosperms, and Dicotyledons native or naturalized in the Washington-Baltimore Area, was published in March 2000. Part II covers the Monocotyledons and completes the preliminary edition of the Checklist, which we hope will prove useful not only in itself but also as a first step toward a new manual for the identification of the Area’s flora. Such a manual is needed to replace the long- outdated and out-of-print Flora of the District of Columbia and Vicinity of Hitchcock and Standley, published in 1919. In the preparation of this part, as with Part I, Shetler has been responsible for the taxonomy and nomenclature and Orli for the database. As with the first part, we are distributing this second part in preliminary form, so that it can be used, criticized, and updated while the two parts are being readied for publication as a single volume. -

3381-001 Donald Sankey Farner Papers Inventory Accession
UNlVERSllY U BRARIJES w UNIVERSITY of WASHI NGTON Spe ial Colle tions 3784 Donald Sankey Farner papers Inventory Accession No: 3381-001 Special Collections Division University of Washington Libraries Box 352900 Seattle, Washington, 98195-2900 USA (206) 543-1929 This document forms part of the Preliminary Guide to the Donald Sankey Farner Papers. To find out more about the history, context, arrangement, availability and restrictions on this collection, click on the following link: http://digital.lib.washington.edu/findingaids/permalink/FarnerDonaldSUA3381/ Special Collections home page: http://www.lib.washington.edu/specialcollections/ Search Collection Guides: http://digital.lib.washington.edu/findingaids/search DONALDS. FARNER Accession No. 3381-88-21 INVENTORY Box Series Folders Dates 1 GENERAL CORRESPONDENCE A 2 1957-60, 1968, 1970-73,1976-87 Abbott, Ian John 1968 Abelson, Philip H. 1980 Abs, Michael 1981 Academic Press Royalty Statement 1977-79 Adam, Hans 1982 Adkisson, C.S. 1979 Ainley, M.G. 1980, 1985 Akesson, Thomas 1979 Akita, Yasukazu n.d. Aldrich, John W. 1946-58, 1961 1968-72 Alexander Von Humboldt-Stiftung 2 1976-85, 1987 Ali, M.A. 1964 Alvarado, Ronald H. 2 1965, n.d. Alvarez, Bonnie 1970-73 Ameel, Donald J. 1961-65 American Elsevier Publishing Co. n.d. American Express 1977, 1984 Amodon, Dean 1947-51, 1961, 1965-66 Amoroso, E.C. 1961-64, 1974-77 Anderson, Berti! G. n.d. Andrewartha, Herbert G. 1959, 1963 Arcese, Peter 1982 Arnold, Arthur P. 1973-74 Aschoff, Jurgen 1965-83 Ashmole, N. Philip 1969-72 Arvey, M. Dale 1949-73 Assenmacher, Ivan 1960-83 American Society of Zoologists 1983 Audubon Conservation Topics West 1980 Austin, O.L. -

Depression, Obesity, Eating Behavior, and Physical Activity
Journal of Obesity Depression, Obesity, Eating Behavior, and Physical Activity Guest Editors: Kristin L. Schneider, Austin S. Baldwin, Devin M. Mann, and Norbert Schmitz Depression, Obesity, Eating Behavior, and Physical Activity Journal of Obesity Depression, Obesity, Eating Behavior, and Physical Activity Guest Editors: Kristin L. Schneider, Austin S. Baldwin, Devin M. Mann, and Norbert Schmitz Copyright © 2012 Hindawi Publishing Corporation. All rights reserved. This is a special issue published in “Journal of Obesity.” All articles are open access articles distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is prop- erly cited. Editorial Board David Allison, USA Alfredo Halpern, Brazil Denis Richard, Canada B. J. Ammori, UK Xu Feng Huang, Australia Robert Ross, Canada Marco Anselmino, Italy Terr y Huang , USA Jonatan R. Ruiz, Sweden Molly S. Bray, USA Gianluca Iacobellis, Canada Jordi Salas-Salvado’, Spain Bernhard Breier, NewZealand Lauren E. Lissner, Sweden Francesco S. Papadia, Italy Eliot Brinton, USA Yannis Manios, Greece J. C. Seidell, TheNetherlands Yvon Chagnon, Canada Claude Marcus, Sweden Gianfranco Silecchia, Italy Karen Charlton, Australia Ron F. Morrison, USA Laurence Tecott, USA Eric Doucet, Canada Michael M. Murr, USA Rob M. Van Dam, Singapore Pietro Forestieri, Italy Tomoo Okada, Japan Youfa Wang, USA Jayne Fulkerson, USA Renato Pasquali, Italy Aron Weller, Israel Jesus´ M. Garagorri, Spain Mark A. Pereira, -

Individual and Organizational Donors
INDIVIDUAL AND ORGANIZATIONAL Mr. Saumya Nandi and Ms. Martha Delgado Edward & Rose Donnell Foundation Dr. Tim D. Noel and Mrs. Joni L. Noel Mr. and Mrs. John A. Edwardson DONORS Orange Crush, LLC Ms. Amberlynne Farashahi Park Avenue Financial Group Trust Mr. and Mrs. Blair Farwell $100,000 and above Mr. and Mrs. Mark J. Parrell The Field Foundation of Illinois Anonymous (4) The Pritzker Pucker Family Foundation Fortune Brands, Inc. Bank of America Mr. Richard Proulx Franklin Philanthropic Foundation BlackEdge Capital Bruce and Diana Rauner Mr. Philip M. Friedmann The Chicago Community Trust The Regenstein Foundation Futures Industry Association Feeding America Mr. and Mrs. Bradley S. Reid Garvey's Office Products Ms. Susan E. Grabin The Rhoades Foundation GCA Services Group, Inc. Hardison Family Foundation Mr. and Mrs. James H. Roth General Iron Industries Charitable Foundation Mr. and Mrs. Raymond L. Harriman Roundy's Foundation Dr. Glenn S. Gerber and Ms. Linda S. Schurman Hillshire Brands Foundation The Satter Family Foundation Gethsemane United Church of Christ Daniel Haerther Living Trust Mr. and Mrs. Travis Schuler Mr. and Mrs. Brent Gledhill Mr. Albert F. Hofeld Mrs. Rose L. Shure Goldberg Kohn, Ltd. Mr. Michael L. Keiser and Mrs. Rosalind Keiser Julie and Brian Simmons Foundation Golub & Company Kraft Foods Group Foundation SmithBucklin Corporation Google, Inc. Ann Lurie Revocable Trust The Smogolski Family 2008 Mr. and Mrs. Andrew M. Gore Polk Bros. Foundation Charitable Lead Trust W.W. Grainger, Inc. Share Our Strength The Telos Group LLC Grand Kids Foundation Mr. William R. Shepard Stanley and Lucy Lopata Charitable Foundation Ms. -

AMICA Bulletin
The www.amica.org AMICA Bulletin Volume 48, Number 6 November/December 2011 Automatic Musical Instrument Collectors’ Association This ad prepared with the help of Rosanna Harris, with thanks ISSN #1533-9726 The AMICA BulleTIn AUToMATIC MUSICAL INSTRUMENT CoLLECToRS' ASSoCIATIoN Published by the Automatic Musical Instrument Collectors’ Asso- Visit the AMICA web site at: http://www.amica.org ciation, a 501(c)(3) non-profit, tax exempt group devoted to the to enter the “Members-Only” portal, restoration, distribution and enjoyment of musical instruments Current User Name: AMICA using perforated paper music rolls and perforated music books. Current password: rewind (to end 2011) AMICA was founded in San Francisco, California in 1963. New password: treadle (from 1 Jan 2012) VoLUME 48, Number 5 November/December 2011 AMICA BULLETIN FEATURES DEADLINES Ads and articles must be received AMICA in England 2011 . by Shirley Nix . 300 on or before the 1st of these ODD AMICA in Pittsburgh 2012 . .by Tim Baxter . 303 months: Nickel Notes . by Matthew Jaro . 304 January July Mechanical Music Today . by Marc Sachnoff . 308 March September Visit to the Popper Showroom . by Q. David Bowers . 330 May November Duo-Art Organ Concertola . by Paul Morris . 333 Bulletins will ordinarily be mailed in Tribute to Larry Givens . .by 3 Friends . 348 the 1st week of the even months, for expected delivery mid-month. COLUMNS Terry Smythe 55 Rowand Avenue President’s Message. 296 Winnipeg, MB, Canada R3J2N6 Vice-President’s Message . 297 204-832-3982 (email preferred) Editorial Observations . 296 [email protected] Letters . 298 80 Years Young . .by Alan Turner . -

Global Distribution of Anaerobic Dichloromethane Degradation Potential
bioRxiv preprint doi: https://doi.org/10.1101/2021.08.30.458270; this version posted August 31, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. 1 Global distribution of anaerobic dichloromethane degradation potential 2 3 Short Title: Anaerobic dichloromethane biodegradation 4 5 Robert W. Murdoch1†, Gao Chen1,2, Fadime Kara Murdoch1,7†, E. Erin Mack5, Manuel I. Villalobos Solis6, 6 Robert L. Hettich6, and Frank E. Löffler1,2,3,4,6* 7 1Center for Environmental Biotechnology, 2Department of Civil and Environmental Engineering, 8 3Department of Microbiology, 4Department of Biosystmes Engineering and Soil Science, University of 9 Tennessee, Knoxville, TN 37996, USA 10 5Corteva Environmental Remediation, Corteva Agriscience, Wilmington, DE 19805, USA 11 6Biosciences Division, Oak Ridge National Laboratory, Oak Ridge, TN 37831, USA 12 † Current address: Battelle Memorial Institute, Columbus, Ohio 43201 13 * Corresponding author: Frank E. Löffler. Center for Environmental Biotechnology, 14 University of Tennessee, 676 Dabney Hall, 1416 Circle Drive, Knoxville, TN 37996-1605, USA 15 Phone: (865) 974 4933 Email: [email protected] 16 Competing Interest Statement: The authors declare no competing interest. 17 Classification: Biological Sciences (Major); Environmental Sciences (Minor) 18 Keywords: Dichloromethane fluxes, Degradation Potential, Bioremediation, Ozone Destruction 19 Abstract 20 Anthropogenic activities and natural processes release dichloromethane (DCM), a toxic chemical with 21 substantial ozone-depleting capacity. Specialized anaerobic bacteria metabolize DCM; however, the 22 genetic basis for this process has remained elusive. Comparative genomics of the three known 23 anaerobic DCM-degrading bacterial species revealed a homologous gene cluster, designated the 24 methylene chloride catabolism (mec) gene cassette, comprising eight to ten genes with predicted 79.6 – 25 99.7% amino acid identity. -

Surname Given Age Date Page Maiden Note Abildua Frank 91 22-Dec D-1 Ables Cary James 14 13-Apr D-1 Full Name Abrahamson Ethel 91 9-Mar D-2 Newman Abramson Rose H
Surname Given Age Date Page Maiden Note Abildua Frank 91 22-Dec D-1 Ables Cary James 14 13-Apr D-1 Full name Abrahamson Ethel 91 9-Mar D-2 Newman Abramson Rose H. 81 2-Feb D-2 Accordini Mary G. 70 20-Dec A-13 Ackerman Frances L. 4-May C-5 Adams Eva 83 3-Apr D-2 Stewart Adams Michael J. 76 28-Jan D-1 Adell Alfred S. 48 5-May B-4 Adoba William J., Sr. 64 10-Jan B-6 Agnew W. Lynn 85 12-Sep B-6 Albaugh James 81 9-Oct E-1 Albee Daniel 62 27-Jul D-1 Albert Henrietta 79 13-Apr D-1 Albright Ralph G. 80 13-Apr D-1 Aldrin Myrtle A. 76 15-Jan D-6 Alexander Daniel L. 37 13-Jun C-5 Alexander W. (Rev.) 69 6-Jan D-2 Alexich Jacov "Jack" 60 26-Dec D-1 Allande Arthur 80 13-Oct D-3 Allande Augustina 79 2-May C-2 Allen James P., Sr. 49 30-Oct D-1 Allen Ernest L. 48 6-Sep B-5 Veteran of the Korean conflict Allen Lizzie 3-Jan A-11 Allen William M. 75 21-Mar C-2 Allen Aryln J. 60 5-Jun F-7 Allen Annie 101 4-Feb D-1 Aller Edward D. 81 11-Jan D-2 Almanza Maria 81 13-Feb D-1 Aloia Frank, Sr. 90 9-Feb C-2 Alongi Samuel 70 5-Jan C-5 Veteran of World War II Alpert Samual 97 15-Dec C-7 Alsdorf Rose Evelyn 52 30-Sep C-3 Full name Alsip Yvetta E. -

Lactoferrin's Anti-Cancer Properties
biomolecules Review Lactoferrin’s Anti-Cancer Properties: Safety, Selectivity, and Wide Range of Action 1, , 2, 1 2 Antimo Cutone * y, Luigi Rosa y , Giusi Ianiro , Maria Stefania Lepanto , Maria Carmela Bonaccorsi di Patti 3 , Piera Valenti 2 and Giovanni Musci 1,* 1 Department of Biosciences and Territory, University of Molise, 86090 Pesche, Italy; [email protected] 2 Department of Public Health and Infectious Diseases, University of Rome La Sapienza, 00185 Rome, Italy; [email protected] (L.R.); [email protected] (M.S.L.); [email protected] (P.V.) 3 Department of Biochemical Sciences, Sapienza University of Rome, 00185 Rome, Italy; [email protected] * Correspondence: [email protected] (A.C.); [email protected] (G.M.) These authors contributed equally to this work. y Received: 25 February 2020; Accepted: 13 March 2020; Published: 15 March 2020 Abstract: Despite recent advances in cancer therapy, current treatments, including radiotherapy, chemotherapy, and immunotherapy, although beneficial, present attendant side effects and long-term sequelae, usually more or less affecting quality of life of the patients. Indeed, except for most of the immunotherapeutic agents, the complete lack of selectivity between normal and cancer cells for radio- and chemotherapy can make them potential antagonists of the host anti-cancer self-defense over time. Recently, the use of nutraceuticals as natural compounds corroborating anti-cancer standard therapy is emerging as a promising tool for their relative abundance, bioavailability, safety, low-cost effectiveness, and immuno-compatibility with the host. In this review, we outlined the anti-cancer properties of Lactoferrin (Lf), an iron-binding glycoprotein of the innate immune defense. -

A Needs Analysis of Adventure Activities in South African National Parks
A needs analysis of adventure activities in South African National Parks ZJ Bosch 21750882 Mini-dissertation submitted in partial fulfillment of the requirements for the degree Magister Artium in Tourism Management at the Potchefstroom Campus of the North-West University Supervisor: Prof P van der Merwe May 2015 ACKNOWLEDGEMENTS The author would like to thank the North-West University (Potchefstroom campus) and the National Research Foundation (NRF) for their financial assistance. Statements and suggestions made in this study are those of the author and should not be regarded as those of the North-West University or the NRF. ACKNOWLEDGEMENTS The following people are thanked for their contribution and support to this study: • Our heavenly father for giving me the talent, patience, strength and persistence to complete my studies. • Prof Peet van der Merwe my leader and supervisor. Thank you for your guidance, advice, support and sense of direction in completing this study. • My family for supporting my dreams and encouraging me to find happiness in everything I do. • Thanks to the people at TREES for providing support when I needed it, especially Dr. Marco Scholtz. • Dr. Suria Ellis for analysing the data from my questionnaires and her constant availability if new comparisons had to be made. • Prof Casper Lessing for his assistance in error searching and editing my bibliography. • A special thanks to SANParks for allowing me to do research on national parks. • Isabel Swart for the grammar editing of this dissertation and translating the abstract/summery into Afrikaans. ABSTRACT Adventure tourism is currently regarded as one of the fastest growing forms of nature-based tourism within the alternative tourism industry. -
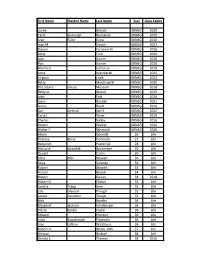
First Name Maiden Name Last Name Year Dues Expire Lynne Bobula
First Name Maiden Name Last Name Year Dues Expire Lynne Bobula 00NBG 2020 Carol Szunyogh Buchanan 00NBG 2020 Jean Fuller Crow 00NBG 2016 Joan M. Deuch 00NBG 2022 Steven Farnsworth 00NBG 2016 Betty Ford 00NBG 2020 Joan Ganim 00NBG 2018 Ron Ganim 00NBG 2018 Martin G. Johnston 00NBG 2019 Anne Kaczmarek 00NBG 2025 Virginia Little 00NBG 2022 Betty Mantkowski 00NBG 2020 The Squire House Museum 00NBG 2018 Betty Jo Niccoli 00NBG 2019 John Pajk 00NBG 2016 Jayne Randall 00NBG 2021 James Roytz 00NBG 2016 Sue Zielinski Schill 00NBG 2022 Carol J. Tener 00NBG 2019 Charles Valley 00NBG 2016 Robert Walker 00NBG 2016 Walter F. Wisnieski 00NBG 2026 Edwin Schmidt 26 Life Theresa Borys Dominick 27 Life Benjamin Pasternak 28 Life Marjorie Kowallek Bruemmer 30 Life Donald Curtis 30 Life Edna Pfeil Stewart 30 Life Anna Valvoda 30 Life Robert Stewart 33 Life Harold Bejcek 34 Life Robert Gaines 34 2018 Robert G. Ehrbar 35 Life Loretta Trapp Kern 35 Life Lois Gleeson Prough 35 Life Louise Davidson Thayer 35 Life Nick Bondra 36 Life Margaret Jackson Echelberger 36 Life Vlasta Kouba Foster 36 Life Edward Hienton 36 Life Luba Kwatkowich Pisanecki 36 Life Jean Laftery Washburn 36 Life Robert V. Webb, DDS 37 Life Weston Birdsall 38 Life Donald L. Thomas 38 2016 First Name Maiden Name Last Name Year Dues Expire Walter G. Broz 39 Life Virginia Klein Gerseny* 39 Life Jack Harper 39 Life George R. Slater 39 Life Ed Spatz 40NBG Life Jean Thomas Vaughan 40 Life Clayton Baum 41 2018 Ruth Kemp DeHaven 41 2017 Doris M.