Fluorite—Illinois’ State Mineral
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Choline for a Healthy Pregnancy
To support healthy for a Healthy weight gain and keep up with the nutritional needs of both mom and Pregnancy the developing baby, CHOLINE additional nutrients are necessary. Nine out of 10 Americans don’t meet the daily recommended choline intake of 550 mg1,2 and it can be challenging to reach this goal even when choosing choline-containing foods like beef, eggs, wheat germ and Brussels sprouts. Choline is particularly important during pregnancy for both mom and baby because it supports healthy brain growth and offers protection against neural tube defects. Women are encouraged to take a prenatal supplement before and during pregnancy to ensure they’re meeting vitamin and mineral recommendations. In fact, the American Medical Association recommends that choline be included in all prenatal vitamins to help ensure women get enough choline to maintain a normal pregnancy.3 Look for a prenatal supplement that contains folic acid, iron, DHA (omega-3s), vitamin D and choline. Consider smart swaps to get the most choline in your diet for a healthy pregnancy, as well as optimal health after baby arrives. PREGNANCY EATING PATTERN* CHOLINE-FOCUSED PREGNANCY EATING PATTERN* 1 1 hard-cooked egg 1 2 cups toasted whole grain oat cereal / 1 large peach 1 cup nonfat milk 1 1 slice whole grain bread /3 cup blueberries 1 1 tablespoon jelly /3 cup sliced banana BREAKFAST 1 cup nonfat milk 1 /2 whole grain bagel 1 whole wheat tortilla 2 tablespoons peanut butter 2 tablespoons peanut butter 1 small apple 1 SNACK 1 /2 large banana /2 cup nonfat vanilla Greek yogurt 2 slices whole grain bread 3 oz. -

Is There an Ideal Diet to Protect Against Iodine Deficiency?
nutrients Review Is There an Ideal Diet to Protect against Iodine Deficiency? Iwona Krela-Ka´zmierczak 1,† , Agata Czarnywojtek 2,3,†, Kinga Skoracka 1,* , Anna Maria Rychter 1 , Alicja Ewa Ratajczak 1 , Aleksandra Szymczak-Tomczak 1, Marek Ruchała 2 and Agnieszka Dobrowolska 1 1 Department of Gastroenterology, Dietetics and Internal Diseases, Poznan University of Medical Sciences, Heliodor Swiecicki Hospital, 60-355 Poznan, Poland; [email protected] (I.K.-K.); [email protected] (A.M.R.); [email protected] (A.E.R.); [email protected] (A.S.-T.); [email protected] (A.D.) 2 Department of Endocrinology, Metabolism and Internal Medicine, Poznan University of Medical Sciences, 60-355 Poznan, Poland; [email protected] (A.C.); [email protected] (M.R.) 3 Department of Pharmacology, Poznan University of Medical Sciences, 60-806 Poznan, Poland * Correspondence: [email protected]; Tel.: +48-665-557-356 or +48-8691-343; Fax: +48-8691-686 † These authors contributed equally to this work. Abstract: Iodine deficiency is a global issue and affects around 2 billion people worldwide, with preg- nant women as a high-risk group. Iodine-deficiency prevention began in the 20th century and started with global salt iodination programmes, which aimed to improve the iodine intake status globally. Although it resulted in the effective eradication of the endemic goitre, it seems that salt iodination did not resolve all the issues. Currently, it is recommended to limit the consumption of salt, which is the main source of iodine, as a preventive measure of non-communicable diseases, such as hypertension or cancer the prevalence of which is increasing. -

Zinc Citrate – a Highly Bioavailable Zinc Source
Wellness Foods Europe THE MAGAZINE FOR NUTRITION, FUNCTIONAL FOODS & BEVERAGES AND SUPPLEMENTS Zinc citrate – a highly bioavailable zinc source Reprint from Wellness Foods Europe issue 3/2014 Wellness Foods Europe Special salts Zinc citrate – a highly bioavailable zinc source Markus Gerhart, Jungbunzlauer Ladenburg GmbH Zinc, the versatile mineral, is about to be- Zinc is a component of about 300 enzymes and come the next star in the minerals catego- 2000 transcriptional factors, and 10 % of the ry. Profiting from its various health benefits human proteome contain zinc-binding motives. and its relatively low cost in use, zinc sales Impairment of intestinal zinc absorption results in supplements have shown a double digit in severe clinical manifestations like skin lesions, growth in 2012 and are starting to catch up developmental retardation, stunted growth and with calcium, magnesium and iron, the cate- immune deficiency. gory leaders. Its importance for human health was empha- sised by the European health claim regu lation, Zinc is an essential transition metal that is where zinc received more positive opinions (18 directly or indirectly involved in a wide varie- in total) than any other mineral. The range of ty of physiological processes. After discover- claims (Table 1) includes, amongst others, im- ing the necessity of zinc for Aspergillus niger, it portant health benefits like immunity, bone took another 100 years before its relevance for health, cognitive function and healthy vision. humans was recognised, when the zinc deficien- These health benefits can be clearly defined and cy syndrome was described for the first time by are easy for the consumer to understand. -

Abstract Spectroscopic Characterization of Fluorite
ABSTRACT SPECTROSCOPIC CHARACTERIZATION OF FLUORITE: RELATIONSHIPS BETWEEN TRACE ELEMENT ZONING, DEFECTS AND COLOR By Carrie Wright This thesis consists of two separate papers on color in fluorite. In the first paper, synthetic fluorites doped with various REEs (10-300 ppm) were analyzed using direct current plasma spectrometry, optical absorption spectroscopy, fluorescence spectrophotometry, and electron paramagnetic resonance spectroscopy before and after receiving 10-25 Mrad of 60Co gamma irradiation. The combined results of these techniques indicate that the irradiation-induced color of the Y-, Gd-, La- and Ce-doped samples are the result of a REE-associated fluorine vacancy that traps two electrons. Divalent samarium may be the cause of the irradiation-induced green color of the Sm- doped sample. In the second paper, fluorite crystals from Bingham, NM, Long Lake, NY, and Westmoreland, NH were similarly investigated to determine the relationship between sectorally zoned trace elements, defects, and color. The results indicate causes of color similar to those in the synthetic samples with the addition of simple F-centers. SPECTROSCOPIC CHARACTERIZATION OF FLUORITE: RELATIONSHIPS BETWEEN TRACE ELEMENT ZONING, DEFECTS AND COLOR A Thesis Submitted to the Faculty of Miami University In partial fulfillment of The requirements for the degree of Master of Science Department of Geology By Carrie Wright Miami University Oxford, OH 2002 Advisor_____________________ Dr. John Rakovan Reader______________________ Dr. Hailiang Dong TABLE OF CONTENTS Chapter 1: Introduction to the cause of color in fluorite 1 Manuscript 1-Chapter 2 29 “Spectroscopic investigation of lanthanide doped CaF2 crystals: implications for the cause of color” Manuscript 2-Chapter 3 95 “Spectroscopic characterization of fluorite from Bingham, NM, Long Lake, NY and Westmoreland, NH: relationships between trace element zoning, defects and color ii TABLE OF FIGURES Chapter 1 Figures 21 Figure 1a. -

Vitamin and Mineral Requirements in Human Nutrition
P000i-00xx 3/12/05 8:54 PM Page i Vitamin and mineral requirements in human nutrition Second edition VITPR 3/12/05 16:50 Page ii WHO Library Cataloguing-in-Publication Data Joint FAO/WHO Expert Consultation on Human Vitamin and Mineral Requirements (1998 : Bangkok, Thailand). Vitamin and mineral requirements in human nutrition : report of a joint FAO/WHO expert consultation, Bangkok, Thailand, 21–30 September 1998. 1.Vitamins — standards 2.Micronutrients — standards 3.Trace elements — standards 4.Deficiency diseases — diet therapy 5.Nutritional requirements I.Title. ISBN 92 4 154612 3 (LC/NLM Classification: QU 145) © World Health Organization and Food and Agriculture Organization of the United Nations 2004 All rights reserved. Publications of the World Health Organization can be obtained from Market- ing and Dissemination, World Health Organization, 20 Avenue Appia, 1211 Geneva 27, Switzerland (tel: +41 22 791 2476; fax: +41 22 791 4857; e-mail: [email protected]). Requests for permis- sion to reproduce or translate WHO publications — whether for sale or for noncommercial distri- bution — should be addressed to Publications, at the above address (fax: +41 22 791 4806; e-mail: [email protected]), or to Chief, Publishing and Multimedia Service, Information Division, Food and Agriculture Organization of the United Nations, 00100 Rome, Italy. The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the World Health Organization and the Food and Agriculture Organization of the United Nations concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. -

Vitamin and Mineral Status in a Vegan Diet
MEDICINE Original Article Vitamin and Mineral Status in a Vegan Diet Cornelia Weikert, Iris Trefflich, Juliane Menzel, Rima Obeid, Alessa Longree, Jutta Dierkes, Klaus Meyer, Isabelle Herter-Aeberli, Knut Mai, Gabriele I. Stangl, Sandra M. Müller, Tanja Schwerdtle, Alfonso Lampen, Klaus Abraham Summary Background: In Germany, public interest in a vegan diet is steadily growing. There are, however, no current data on the macro- and micronutrient status of vegans. Methods: In a cross-sectional study entitled “The Risks and Benefits of a Vegan Diet” (RBVD), we investigated the dietary intake, basic laboratory parameters, vitamin status, and trace-element status of 36 vegans and 36 persons on an omnivorous diet. Each group consisted of 18 men and 18 women aged 30–60. Results: Nearly all the vegans and one-third of the persons on a mixed diet had consumed supplements in the previous 4 weeks. Vegans and non- vegans had similar energy intake but differed in the intake of both macronutrients (e.g., dietary fiber) and micronutrients (e.g., vitamins B12, B2, D, E, and K, as well as folate, iodine, and iron). There were no intergroup differences in the biomarkers of vitamin B12, vitamin D, or iron status. The ferritin values and blood counts indicated iron deficiency in four vegans and three non-vegans. Measurements in 24-hour urine samples revealed lower calcium excretion and markedly lower iodine excretion in vegans compared to non-vegans; in one-third of the vegans, iodine excretion was lower than the WHO threshold value (<20 µg/L) for severe iodine deficiency. Conclusion: Vitamin B12 status was similarly good in vegans and non-vegans, even though the vegans consumed very little dietary B12. -

Human Vitamin and Mineral Requirements
Human Vitamin and Mineral Requirements Report of a joint FAO/WHO expert consultation Bangkok, Thailand Food and Agriculture Organization of the United Nations World Health Organization Food and Nutrition Division FAO Rome The designations employed and the presentation of material in this information product do not imply the expression of any opinion whatsoever on the part of the Food and Agriculture Organization of the United Nations concerning the legal status of any country, territory, city or area or of its authorities, or concern- ing the delimitation of its frontiers or boundaries. All rights reserved. Reproduction and dissemination of material in this information product for educational or other non-commercial purposes are authorized without any prior written permission from the copyright holders provided the source is fully acknowledged. Reproduction of material in this information product for resale or other commercial purposes is prohibited without written permission of the copyright holders. Applications for such permission should be addressed to the Chief, Publishing and Multimedia Service, Information Division, FAO, Viale delle Terme di Caracalla, 00100 Rome, Italy or by e-mail to [email protected] © FAO 2001 FAO/WHO expert consultation on human vitamin and mineral requirements iii Foreword he report of this joint FAO/WHO expert consultation on human vitamin and mineral requirements has been long in coming. The consultation was held in Bangkok in TSeptember 1998, and much of the delay in the publication of the report has been due to controversy related to final agreement about the recommendations for some of the micronutrients. A priori one would not anticipate that an evidence based process and a topic such as this is likely to be controversial. -
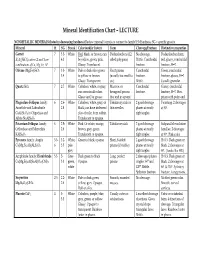
Mineral Identification Chart – LECTURE
Mineral Identification Chart – LECTURE NONMETALLIC MINERALS (listed in decreasing hardness) Review mineral formula to connect to family! H=Hardness; SG = specific gravity Mineral H SG Streak Color (and/or luster) Form Cleavage/Fracture Distinctive properties Garnet 7 3.5- White Red, black, or brown; can Dodecahedrons (12- No cleavage. Dodecahedron form, X3Y2(SiO4)3 where X and Y are 4.3 be yellow, green, pink. sided polygons) Brittle. Conchoidal red, glassy, conchoidal combinations of Ca, Mg, Fe, Al Glassy. Translucent. fracture. fracture, H=7. Olivine (Mg,Fe)2SiO4 7 3.3- White Pale or dark olive green Short prisms Conchoidal Green, conchoidal 3.4 to yellow or brown. (usually too small to fracture. fracture, glassy, H=7. Glassy. Transparent. see). Brittle. Usually granular. Quartz SiO2 7 2.7 White Colorless, white, or gray; Massive; or Conchoidal Glassy, conchoidal can occur in all colors. hexagonal prisms fracture. fracture, H=7. Hex. Glassy and/or greasy. that end in a point. prism with point end. Plagioclase Feldspar family: 6 2.6- White Colorless, white, gray, or Tabular crystals or 2 good cleavage Twinning. 2 cleavages Anorthite and Labradorite 2.8 black; can have iridescent thin needles planes at nearly at 90°. CaAl2Si2O8 to Oligoclase and play of color from within. right angles. Albite NaAlSi3O8 Translucent to opaque. Potassium Feldspar family: 6 2.5- White Pink. Or white, orange, Tabular crystals 2 good cleavage Subparallel exsolution Orthoclase and Microcline 2.6 brown, gray, green. planes at nearly lamellae. 2 cleavages KAlSi3O8 Translucent to opaque. right angles. at 90°. Pink color. Pyroxene family: Augite 5.5- 3.2- White, Green to black; opaque. -

Nutritional Value of Spirulina and Its Use in the Preparation of Some Complementary Baby Food Formulas
Available online at http://journal-of-agroalimentary.ro Journal of Agroalimentary Processes and Journal of Agroalimentary Processes and Technologies Technologies 2014, 20(4), 330-350 Nutritional value of spirulina and its use in the preparation of some complementary baby food formulas Ashraf M. Sharoba Food Sci. Dept., Fac. of Agric., Moshtohor, Benha Univ., Egypt Received: 26 September 2014; Accepted:03 Octomber 2014 .____________________________________________________________________________________ Abstract In this study use the spirulina which is one of the blue-green algae rich in protein 62.84% and contains a high proportion of essential amino acids (38.46% of the protein) and a source of naturally rich in vitamins especially vitamin B complex such as vitamin B12 (175 µg / 10 g) and folic acid (9.92 mg / 100 g), which helps the growth and nutrition of the child brain, also rich in calcium and iron it containing (922.28 and 273.2 mg / 100 g, respectively) to protect against osteoporosis and blood diseases as well as a high percentage of natural fibers. So, the spirulina is useful and necessary for the growth of infants and very suitable for children, especially in the growth phase, the elderly and the visually appetite. It also, helps a lot in cases of general weakness, anemia and chronic constipation. Spirulina contain an selenium element (0.0393 mg/100 g) and many of the phytopigments such as chlorophyll and phycocyanin (1.56% and 14.647%), and those seen as a powerful antioxidant. Finally, spirulina called the ideal food for mankind and the World Health Organization considered its "super food" and the best food for the future because of its nutritional value is very high. -

Winter 1998 Gems & Gemology
WINTER 1998 VOLUME 34 NO. 4 TABLE OF CONTENTS 243 LETTERS FEATURE ARTICLES 246 Characterizing Natural-Color Type IIb Blue Diamonds John M. King, Thomas M. Moses, James E. Shigley, Christopher M. Welbourn, Simon C. Lawson, and Martin Cooper pg. 247 270 Fingerprinting of Two Diamonds Cut from the Same Rough Ichiro Sunagawa, Toshikazu Yasuda, and Hideaki Fukushima NOTES AND NEW TECHNIQUES 281 Barite Inclusions in Fluorite John I. Koivula and Shane Elen pg. 271 REGULAR FEATURES 284 Gem Trade Lab Notes 290 Gem News 303 Book Reviews 306 Gemological Abstracts 314 1998 Index pg. 281 pg. 298 ABOUT THE COVER: Blue diamonds are among the rarest and most highly valued of gemstones. The lead article in this issue examines the history, sources, and gemological characteristics of these diamonds, as well as their distinctive color appearance. Rela- tionships between their color, clarity, and other properties were derived from hundreds of samples—including such famous blue diamonds as the Hope and the Blue Heart (or Unzue Blue)—that were studied at the GIA Gem Trade Laboratory over the past several years. The diamonds shown here range from 0.69 to 2.03 ct. Photo © Harold & Erica Van Pelt––Photographers, Los Angeles, California. Color separations for Gems & Gemology are by Pacific Color, Carlsbad, California. Printing is by Fry Communications, Inc., Mechanicsburg, Pennsylvania. © 1998 Gemological Institute of America All rights reserved. ISSN 0016-626X GIA “Cut” Report Flawed? The long-awaited GIA report on the ray-tracing analysis of round brilliant diamonds appeared in the Fall 1998 Gems & Gemology (“Modeling the Appearance of the Round Brilliant Cut Diamond: An Analysis of Brilliance,” by T. -

Mineralogy and Chemistry of Rare Earth Elements in Alkaline Ultramafic Rocks and Fluorite in the Western Kentucky Fluorspar District Warren H
Mineralogy and Chemistry of Rare Earth Elements in Alkaline Ultramafic Rocks and Fluorite in the Western Kentucky Fluorspar District Warren H. Anderson Report of Investigations 8 doi.org/10.13023/kgs.ri08.13 Series XIII, 2019 Cover Photo: Various alkaline ultramafic rocks showing porphyritic, brecciated, and aphanitic textures, in contact with host limestone and altered dike texture. From left to right: • Davidson North dike, Davidson core, YH-04, 800 ft depth. Lamprophyre with calcite veins, containing abundant rutile. • Coefield area, Billiton Minner core BMN 3. Intrusive breccia with lamprophyric (al- nöite) matrix. • Maple Lake area, core ML-1, 416 ft depth. Lamprophyre intrusive. • Maple Lake area, core ML-2, 513 ft depth. Lamprophyre (bottom) in contact with host limestone (top). Kentucky Geological Survey University of Kentucky, Lexington Mineralogy and Chemistry of Rare Earth Elements in Alkaline Ultramafic Rocks and Fluorite in the Western Kentucky Fluorspar District Warren H. Anderson Report of Investigations 8 doi.org/10.13023/kgs.ri08.13 Series XIII, 2019 Our Mission The Kentucky Geological Survey is a state-supported research center and public resource within the University of Kentucky. Our mission is to sup- port sustainable prosperity of the commonwealth, the vitality of its flagship university, and the welfare of its people. We do this by conducting research and providing unbiased information about geologic resources, environmen- tal issues, and natural hazards affecting Kentucky. Earth Resources—Our Common Wealth www.uky.edu/kgs © 2019 University of Kentucky For further information contact: Technology Transfer Officer Kentucky Geological Survey 228 Mining and Mineral Resources Building University of Kentucky Lexington, KY 40506-0107 Technical Level General Intermediate Technical Statement of Benefit to Kentucky Rare earth elements are used in many applications in modern society, from cellphones to smart weapons systems. -

THE COMPOUND for IMMUNITY SUPPORT Nutritional Supplements & Overall Zinc Benefits
www.vertellus.com ZINC COMPLEXES THE COMPOUND FOR IMMUNITY SUPPORT Nutritional Supplements & Overall Zinc Benefits ZINC Zinc is a trace element that is necessary for a healthy immune system. A lack of zinc can make a person more susceptible to disease and illness. COMPLEXES It is responsible for a number of functions in the human body, and it helps stimulate the activity of at least 100 different enzymes. Only a small intake of The compound for immunity support. zinc is necessary to reap the benefits. Zinc is vital for a healthy immune system, zinc-deficient persons experience increased correctly synthesizing DNA, promoting healthy growth during childhood, and susceptibility to a variety of pathogens. healing wounds. According to the European Journal of Immunology, the human body needs zinc to activate T lymphocytes (T cells). According to a study published in the American Journal of Clinical Nutrition, “zinc-deficient persons experience increased susceptibility to a variety of pathogens.” Research conducted at the University of Toronto and published in the journal Neuron suggested that zinc has a crucial role in regulating how neurons communicate with one another, affecting how memories are formed and how we learn. Zinc plays a role in maintaining skin integrity and structure. Patients experiencing chronic wounds or ulcers often have deficient zinc metabolism and lower serum zinc levels. Zinc is often used in skin creams for treating diaper rash or other skin irritations. A Swedish study that analysed zinc in wound healing concluded, “topical zinc may stimulate leg ulcer healing by enhancing re-epithelialization, decreasing inflammation and bacterial growth.