LSNY Transactions V. 9, 1978
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
CPB1 C10 WEB.Pdf
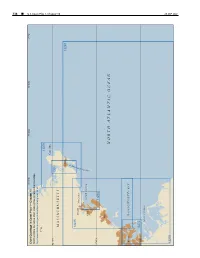
338 ¢ U.S. Coast Pilot 1, Chapter 10 Chapter 1, Pilot Coast U.S. 70°45'W 70°30'W 70°15'W 71°W Chart Coverage in Coast Pilot 1—Chapter 10 NOAA’s Online Interactive Chart Catalog has complete chart coverage http://www.charts.noaa.gov/InteractiveCatalog/nrnc.shtml 71°W 13279 Cape Ann 42°40'N 13281 MASSACHUSETTS Gloucester 13267 R O B R A 13275 H Beverly R Manchester E T S E C SALEM SOUND U O Salem L G 42°30'N 13276 Lynn NORTH ATLANTIC OCEAN Boston MASSACHUSETTS BAY 42°20'N 13272 BOSTON HARBOR 26 SEP2021 13270 26 SEP 2021 U.S. Coast Pilot 1, Chapter 10 ¢ 339 Cape Ann to Boston Harbor, Massachusetts (1) This chapter describes the Massachusetts coast along and 234 miles from New York. The entrance is marked on the northwestern shore of Massachusetts Bay from Cape its eastern side by Eastern Point Light. There is an outer Ann southwestward to but not including Boston Harbor. and inner harbor, the former having depths generally of The harbors of Gloucester, Manchester, Beverly, Salem, 18 to 52 feet and the latter, depths of 15 to 24 feet. Marblehead, Swampscott and Lynn are discussed as are (11) Gloucester Inner Harbor limits begin at a line most of the islands and dangers off the entrances to these between Black Rock Danger Daybeacon and Fort Point. harbors. (12) Gloucester is a city of great historical interest, the (2) first permanent settlement having been established in COLREGS Demarcation Lines 1623. The city limits cover the greater part of Cape Ann (3) The lines established for this part of the coast are and part of the mainland as far west as Magnolia Harbor. -

Historical Collections of the Topsfield Historical Society
Digitized by the Internet Archive in 2015 https://archive.org/details/historicalcollec26unse r. ; V:i , M 4 \ 1790. March, SALEM. Magazine, HOUSE, "Massachusetts TOWN AND the in HOUSE published COURT Hill, THE S. OF by VIEW engraving the From IVIUj THE HISTORICAL COLLECTIONS OF THE TOPSFIELD HISTORICAL SOCIETY VOLUME XXVI 1921 TOPSFIELD MASS. PUBLISHED BY THE SOCIETY 1921 GEORGE FRANCIS DOW Editor THE PERKINS PRESS MASS. CONTENTS VIEW OF THE COURT HOUSE, SALEM IN 1790 - - Frontispiece OFFICERS OF THE SOCIETY, 1920 iv ANNUAL REPORT OF THE SECRETARY FOR THE YEAR ENDING DEC. 31, 1920 V ANNUAL REPORT OF THE TREASURER FOR THE YEAR ENDING DEC. 31, 1920 vii - - ANNUAL REPORT ON THE BUILDING FUND - . viii ESSEX COUNTY IN THE MASSACHUSETTS BAY COLONY AS DE- SCRIBED BY EARLY TRAVELERS. COMMUNICATED BY GEORGE FRANCIS DOW (continued) 1 THE STORY OF A PEABODY HOUSE AND ITS NEIGHBORHOOD. BY CHARLES JOEL PEABODY 113 RECORDS OF MEETINGS OF THE CITIZENS AND COMMITTEE OF ARRANGEMENTS FOR THE CELEBRATION OF THE TWO HUNDREDTH ANNIVERSARY OF THE SETTLEMENT OF THE TOWN OF TOPSFIELD, 1850. COMMUNICATED BY LEONE P. WELCH 121 NEWSPAPER ITEMS RELATING TO TOPSFIELD, COPIED BY GEORGE FRANCIS DOW 128 TOPSFIELD VITAL STATISTICS, 1920 141 CHRONOLOGY OF EVENTS 1920, 144 BUILDINGS CONSTRUCTED, 1920 144 OFFICERS OF THE TOPSFIELD HISTORICAL SOCIETY 1920 President Charles Joel Peabody Vice-President Thomas Emerson Proctor Secretary and Treasurer George Francis Dow Curator Albert M. Dodge Board of Directors Charles Joel Peabody, ex-officio Thomas Emerson Proctor, ex-officio George Francis Dow, ex-officio W. Pitman Gould Isaac H. Sawyer Leone P. Welch Arthur H. -

Pirates and Buccaneers of the Atlantic Coast
ITIG CC \ ',:•:. P ROV Please handle this volume with care. The University of Connecticut Libraries, Storrs Digitized by the Internet Archive in 2011 with funding from Lyrasis Members and Sloan Foundation http://www.archive.org/details/piratesbuccaneerOOsnow PIRATES AND BUCCANEERS OF THE ATLANTIC COAST BY EDWARD ROWE SNOW AUTHOR OF The Islands of Boston Harbor; The Story of Minofs Light; Storms and Shipwrecks of New England; Romance of Boston Bay THE YANKEE PUBLISHING COMPANY 72 Broad Street Boston, Massachusetts Copyright, 1944 By Edward Rowe Snow No part of this book may be used or quoted without the written permission of the author. FIRST EDITION DECEMBER 1944 Boston Printing Company boston, massachusetts PRINTED IN THE UNITED STATES OF AMERICA IN MEMORY OF MY GRANDFATHER CAPTAIN JOSHUA NICKERSON ROWE WHO FOUGHT PIRATES WHILE ON THE CLIPPER SHIP CRYSTAL PALACE PREFACE Reader—here is a volume devoted exclusively to the buccaneers and pirates who infested the shores, bays, and islands of the Atlantic Coast of North America. This is no collection of Old Wives' Tales, half-myth, half-truth, handed down from year to year with the story more distorted with each telling, nor is it a work of fiction. This book is an accurate account of the most outstanding pirates who ever visited the shores of the Atlantic Coast. These are stories of stark realism. None of the arti- ficial school of sheltered existence is included. Except for the extreme profanity, blasphemy, and obscenity in which most pirates were adept, everything has been included which is essential for the reader to get a true and fair picture of the life of a sea-rover. -

A Springtime Exploration of Essex County's Coastal Islands, With
bo33-1:BO32-1.qxd 6/2/2011 6:26 AM Page 12 A Springtime Exploration of Essex County’s Coastal Islands, with Notes on Their Historical Use by Colonially Nesting Birds Jim Berry For over thirty years I have lived on the North Shore of Massachusetts without a boat and have long wondered what colonially nesting birds, in what numbers, have nested on the many islands along the Essex County coast. All I knew was that large gulls and cormorants nest on some of them, that terns used to nest on them, and that herons have used at least three of them, but beyond that I didn’t know many details. In 2004 I got a chance to learn more when I found out that my friends Mary Capkanis and Dave Peterson had acquired a boat, and that Mary had obtained a pilot’s license. Both are longtime birders, and both have experience surveying waterbird colonies in various parts of the U.S. Finally, I had the means to do some island- hopping with friends who were serious about surveying for nesting birds. We made three outings, on May 12, May 14, and May 31. Linda Pivacek accompanied us on the third trip. We were unable to get out in June and of course needed more trips, later in the nesting season, to complete even a preliminary census. But in those three days we visited (with very few landings) most of the 30+ islands between Rockport and Nahant that are large enough to support nesting birds. I had three goals for these trips: (1) to see where gulls and cormorants are nesting and in roughly what numbers, and whether terns still nest on any of the islands; (2) to find out whether herons are currently nesting on any islands other than Kettle, off Manchester; and (3) to look for evidence of nesting by other species, such as Common Eiders (Somateria mollissima), which have increased dramatically as nesters in Boston Harbor, and Great Cormorants (Phalacrocorax carbo), Black Guillemots (Cepphus grylle), and American Oystercatchers (Haematopus palliatus), for which there are no documented nesting records in Essex County. -

Table 1. Results of the 1994-95 Massachusetts Coastal Colonial
Table 1. Results of the 1994-95 Massachusetts Coastal Colonial Waterbird Inventory showing estimated numbers of pairs for all species at all colonies located in the coastal zone. An asterisk (*) indicates "count not taken." Data compiled by B.G. Blodget, Massachusetts Division of Fisheries and Wildlife. COLONY SPECIES NO. OF PAIRS CENSUS REPORTING NUMBER COLONY NAME, TOWN CODE 1994 1995 METHOD1 AUTHORITY 324007.0 Woodbridge I., Newburyport COTE 65 62 ACG FWS 324007.1 Blackwater R. Group, Salisbury 0 0 TTOR 324007.2 Chaces I., Newbury COTE 14 0 ACG FWS HEGU 0 1 NCG FWS 324008.0 Plum I. R. Group, Newbury COTE 34 30 NCG FWS 324009.0 Parker R. Group, Newbury COTE 10 15 NCG FWS 324009.1 Plum I. Beach, Newbury-Rowley-Ip LETE 32 19 NCG FWS 324010.0 Roger I., Ipswich COTE 6 2 ACB ECG 324010.1 Stage I.-Cross Hill F., Ipswich * 0 FWS 324010.2 Bagwell I., Ipswich COTE 8 8 ACB ECG 324010.3 Rowley Salt Marshes, Rowley COTE 3 2 ACB ECG 324010.4 Lord's I., Ipswich COTE 25 20 ACB ECG 324010.5 Ipswich Salt Marshes, Ipswich COTE 13 13 ACB ECG 324011.0 Crane Beach, Ipswich LETE 30 75 NCG TTOR 324012.0 Tenpound I., Gloucester HEGU 75 * NCG DFW GBBG 70 * NCG DFW 324013.0 Norman's Woe, Gloucester DCCO 199 * NCG DFW HEGU 76 * NCG DFW TABLE 1... 2 COLONY SPECIES NO. OF PAIRS CENSUS REPORTING NUMBER COLONY NAME, TOWN CODE 1994 1995 METHOD AUTHORITY GBBG 4 * NCG DFW 324014.0 Kettle I., Gloucester GREG 42 * NCG DFW SNEG 207 * NCG DFW LBHE 6 * NCG DFW BCNH 7 * NCG DFW GLIB 35 * NCG DFW HEGU 388 * NCG DFW GBBG 146 * NCG DFW 324015.0 Graves I., Manchester HEGU 97 * NCG DFW GBBG 74 * NCG DFW 324016.0 Great Egg Rock, Manchester DCCO 124 * NCB DFW GBBG 2 * NCB DFW 324017.0 Dry Salvages, Rockport DCCO * 19 NCG DFW 324018.0 Straitsmouth I., Rockport HEGU 23 * NCG MAS GBBG 143 * NCG MAS 324019.0 Thacher I., Rockport BCNH 1 1 NCG DFW GLIB 0 1 AEG DFW HEGU 1,185 1,359 NCG FWS TABLE 1.. -

Summary of 2006 Census of American Oystercatchers in Massachusetts
SUMMARY OF 2006 CENSUS OF AMERICAN OYSTERCATCHERS IN MASSACHUSETTS Compiled by: Scott M. Melvin Natural Heritage and Endangered Species Program Massachusetts Division of Fisheries and Wildlife Westborough, MA 01581 August 2007 SUMMARY OF 2006 CENSUS OF AMERICAN OYSTERCATCHERS IN MASSACHUSETTS INTRODUCTION This report summarizes data collected during a statewide census of the American Oystercatcher (Haematopus palliatus) in Massachusetts during the 2006 breeding season. This census was conducted by a statewide network of cooperating agencies and organizations. The American Oystercatcher is a large, strikingly colored shorebird that nests on coastal beaches along the Atlantic Coast from Maine to Florida. Although the species has expanded its range and increased in abundance in New England over the past 50 years, its range extension northward along the Atlantic Coast may well be a recolonization of formerly occupied habitat (Forbush 1912). On a continental scale, the American Oystercatcher is one of the most uncommon species of breeding shorebirds in North America and has been designated a Species of High Concern in the United States Shorebird Conservation Plan (Brown et. al. 2001). METHODS Data on American Oystercatcher abundance, distribution, and reproductive success were collected by a coast-wide group of cooperators that included full-time and seasonal biologists and coastal waterbird monitors, beach managers, researchers, and volunteers. This is the same group that conducts annual censuses of Piping Plovers and terns in Massachusetts (Melvin 2007, Mostello 2007). Observers censused adult oystercatchers during or as close as possible to the designated census period of 22 - 31 May 2006, in order to minimize double-counting of birds that might move between multiple sites during the breeding season. -

Fishermen of the Atlantic
The University of Maine DigitalCommons@UMaine History of Maine Fisheries Special Collections 1920 Fishermen of the Atlantic Fishing Masters' Association Follow this and additional works at: https://digitalcommons.library.umaine.edu/fisheries Part of the Agricultural and Resource Economics Commons, and the Aquaculture and Fisheries Commons Repository Citation Fishing Masters' Association, "Fishermen of the Atlantic" (1920). History of Maine Fisheries. 144. https://digitalcommons.library.umaine.edu/fisheries/144 This Book is brought to you for free and open access by DigitalCommons@UMaine. It has been accepted for inclusion in History of Maine Fisheries by an authorized administrator of DigitalCommons@UMaine. For more information, please contact [email protected]. BOSTON yy. - m Fairbanks-Morse Type "C-0" INSURANCE Marine Oil Engine COMPANY Incorporated 1873 Established by Service Capital, $1,000,000 Surplus, $2,000,000 -- i I INSURANCE ON FISHING I L VESSELS AND OUTFITS A SPECIALTY FIRE, MARINE, WAR, YACHT, AUTOMOBILE and TOURISTS' BAGGAGE INSURANCE XRi& HOME OFFICE, 87 KILBY STREET FAIRBANKS, MORSE & CO. 1 BOSTON, MASSACHUSETTS BOSTON NEW YORK BALTIMORE New York Office, 66 Beaver Street New York ADMINISTRATION BUILDING, BOSTON FISH PIER, BOSTON, MASS. JOHN NAGLE. President R. J. AHEARN, Treasurer JOHN NAGLE, JR., Vice-president GEORGE I,. POLAND, Secretary JOHN NAGLE CO., I~C. Fishing Masters' Wholesale and Commission Dealers in all kinds of Association Fresh Fish of the Our Specialty is supplying Fresh and Frozen Bait of All Kinds to Fishermen Administration Building Atlantic Boston Fish Pier, Boston, Mass. Telephones, Port Hill 5650 and 5651 ,\ .Il.\h'ITATA OF IKFOIChl;\TTO?\', TSSUED ANNTTL\T,LY, COI\"l'A\IN IS(: THE ONLY Coi3IJT~I4Yl"l'< LIST OF FISHING VESSELS OF I1OSTON, (;L0TT('ESl1lClt, 1'I:O\'IN('lCTO\VN, CHATH.\.\I, XliCIV l~l~~l)FOI~l),l'OItTTAL\KI), AfIiC. -

Survey of Coastal Nesting Colonies of Cormorants, Gulls, Night-Herons, Egrets, and Ibises in Massachusetts, 2006-08
Survey of Coastal Nesting Colonies of Cormorants, Gulls, Night-Herons, Egrets, and Ibises in Massachusetts, 2006-08 Final Report Compiled by Scott M. Melvin Natural Heritage and Endangered Species Program Massachusetts Division of Fisheries and Wildlife Westborough, MA 01581 Survey of Coastal Nesting Colonies of Cormorants, Gulls, Night-Herons, Egrets, and Ibises in Massachusetts, 2006-08 Final Report Compiled by Scott M. Melvin Natural Heritage and Endangered Species Program Massachusetts Division of Fisheries and Wildlife Westborough, MA 01581 20 December 2010 Cover photo credits (clockwise from upper left): Double-crested Cormorants, Scott Melvin; Black-crowned Night-Herons, Jack Swedberg; Herring Gulls, Bill Byrne; Snowy Egret, Dusty Perin 2 ABSTRACT This report summarizes results of a coastwide survey of colonial waterbirds nesting in Massachusetts that was conducted during May and June, 2006-08. Species surveyed included Double-crested Cormorant (Phalacrocorax auritus), Great Egret (Casmerodius albus), Snowy Egret (Egretta thula), Little Blue Heron (Egretta caerulea), Cattle Egret (Bubulcus ibis), Black-crowned Night-Heron (Nycticorax nycticorax), Glossy Ibis (Plegadis falcinellus), Laughing Gull (Larus atricilla), Herring Gull (L. argentatus), and Great Black-backed Gull (L. marinus). A total of 96 known or potential colony sites, including all significant colony sites that were surveyed in the last comprehensive survey in 1994-1995, were surveyed during May or June in 2006 or 2007. One additional historic site, Clarks Island in Plymouth, was surveyed by boat on 5 June 2008. Additional counts of roof-nesting gulls at selected sites in 2006 and 2007 were reported pursuant to depredation permits. Surveys at most colonies consisted of total nest counts made by observers on the ground. -

Population Changes in New England Seabirds Author(S): William H
Population Changes in New England Seabirds Author(s): William H. Drury Source: Bird-Banding , Autumn, 1973, Vol. 44, No. 4 (Autumn, 1973), pp. 267-313 Published by: Wiley on behalf of Association of Field Ornithologists Stable URL: https://www.jstor.org/stable/4511982 JSTOR is a not-for-profit service that helps scholars, researchers, and students discover, use, and build upon a wide range of content in a trusted digital archive. We use information technology and tools to increase productivity and facilitate new forms of scholarship. For more information about JSTOR, please contact [email protected]. Your use of the JSTOR archive indicates your acceptance of the Terms & Conditions of Use, available at https://about.jstor.org/terms Wiley and Association of Field Ornithologists are collaborating with JSTOR to digitize, preserve and extend access to Bird-Banding This content downloaded from 128.253.96.84 on Mon, 16 Nov 2020 00:45:42 UTC All use subject to https://about.jstor.org/terms POPULATION CHANGES IN NEW ENGLAND SEABIRDS By WILLIAM H. DRURY This paper reviews the historical changes in seabird populations between New York City and the Grand Manan Archipelago,New Brunswick, after 1900. Special attention has been given to species that nest on treeless outer islands of the coast of Maine. In a com- panion paper Nisbet (1973) treats the history of the tern popula- tions, concentrating on the area between Cape Cod and Long Island. The purpose of this paper is to compare the results of a pilot survey made in the last few years to the population changes of the last 75 years. -

Survey of Coastal Nesting Colonies of Cormorants, Gulls, Night-Herons, Egrets, and Ibises in Massachusetts, 2006-08
Survey of Coastal Nesting Colonies of Cormorants, Gulls, Night-Herons, Egrets, and Ibises in Massachusetts, 2006-08 Final Report Compiled by Scott M. Melvin Natural Heritage and Endangered Species Program Massachusetts Division of Fisheries and Wildlife Westborough, MA 01581 Survey of Coastal Nesting Colonies of Cormorants, Gulls, Night-Herons, Egrets, and Ibises in Massachusetts, 2006-08 Final Report Compiled by Scott M. Melvin Natural Heritage and Endangered Species Program Massachusetts Division of Fisheries and Wildlife Westborough, MA 01581 20 December 2010 Cover photo credits (clockwise from upper left): Double-crested Cormorants, Scott Melvin; Black-crowned Night-Herons, Jack Swedberg; Herring Gulls, Bill Byrne; Snowy Egret, Dusty Perin 2 ABSTRACT This report summarizes results of a coastwide survey of colonial waterbirds nesting in Massachusetts that was conducted during May and June, 2006-08. Species surveyed included Double-crested Cormorant (Phalacrocorax auritus), Great Egret (Casmerodius albus), Snowy Egret (Egretta thula), Little Blue Heron (Egretta caerulea), Cattle Egret (Bubulcus ibis), Black-crowned Night-Heron (Nycticorax nycticorax), Glossy Ibis (Plegadis falcinellus), Laughing Gull (Larus atricilla), Herring Gull (L. argentatus), and Great Black-backed Gull (L. marinus). A total of 96 known or potential colony sites, including all significant colony sites that were surveyed in the last comprehensive survey in 1994-1995, were surveyed during May or June in 2006 or 2007. One additional historic site, Clarks Island in Plymouth, was surveyed by boat on 5 June 2008. Additional counts of roof-nesting gulls at selected sites in 2006 and 2007 were reported pursuant to depredation permits. Surveys at most colonies consisted of total nest counts made by observers on the ground. -

Wrecks Around Nantucket : Since the Settlement of the Island, and The
1 ALLEN COUNTY PUBLIC LIBRARY GC 3 1833 06500 4894 974.402 N15GA WRECKS AROUND NANTUCKET Compiled by ARTHUR H. GARDNER 1915 With Additional Records to 1954 I WRECKS AROUND NANTUCKET Since the settlement of the island, and the incidents connected therewith, embracing over seven hundred vessels. Compiled by ARTHUR H. GARDNER. Reynolds Printing, Inc., New Bedford, Mass. Copyright 1915 by Arthur H. Gardner. Copyright March 11, 1943 by Grace Brown Gardner Introduction to First Edition, In presenting this book to the public, it may be well to say a few words in regard to the geographical position of the island, the nature of the coast, and the vast extent of dangerous shoals contigu¬ ous and stretching seaward for many leagues, which have ever proved a terror to mariners, and upon which so many noble vessels have “wound up their logs” for all time, consigning myriads of human be¬ ings to a watery grave. The island of Nantucket is situated some thirty miles southeast of Massachusetts, is fifteen miles in length, with an average breadth of four or five, and presents a coast line of about seventy-five miles. Owing to the peculiar shape of the island, and the indentures made by the harbor, the coast line, especially on the northern side, is exceedingly irregular. A light sandy beach extends around the island, and with the exception of a small reef in Muskeget Channel and a few isolated ones in the immediate vicinity of the shore on the north side of the island and Tuckernuck, the coast is entirely clear of rocks. -

Sustainable Development of Maritime Cultural Heritage in the Gulf of Maine Stefan Claesson University of New Hampshire, Durham
University of New Hampshire University of New Hampshire Scholars' Repository Doctoral Dissertations Student Scholarship Spring 2008 Sustainable development of maritime cultural heritage in the Gulf of Maine Stefan Claesson University of New Hampshire, Durham Follow this and additional works at: https://scholars.unh.edu/dissertation Recommended Citation Claesson, Stefan, "Sustainable development of maritime cultural heritage in the Gulf of Maine" (2008). Doctoral Dissertations. 419. https://scholars.unh.edu/dissertation/419 This Dissertation is brought to you for free and open access by the Student Scholarship at University of New Hampshire Scholars' Repository. It has been accepted for inclusion in Doctoral Dissertations by an authorized administrator of University of New Hampshire Scholars' Repository. For more information, please contact [email protected]. SUSTAINABLE DEVELOPMENT OF MARITIME CULTURAL HERITAGE IN THE GULF OF MAINE BY STEFAN CLAESSON B.A., Boston University, 1992 M.A., Texas A&M University, 1998 DISSERTATION Submitted to the University of New Hampshire in Partial Fulfillment of the Requirements for the Degree of Doctor of Philosophy in Natural Resources and Environmental Studies May, 2008 UMI Number: 3308369 Copyright 2008 by Claesson, Stefan All rights reserved. INFORMATION TO USERS The quality of this reproduction is dependent upon the quality of the copy submitted. Broken or indistinct print, colored or poor quality illustrations and photographs, print bleed-through, substandard margins, and improper alignment can adversely affect reproduction. In the unlikely event that the author did not send a complete manuscript and there are missing pages, these will be noted. Also, if unauthorized copyright material had to be removed, a note will indicate the deletion.