Neural and Receptor Cochlear Potentials Obtained by Transtympanic Electrocochleography in Auditory Neuropathy Q
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
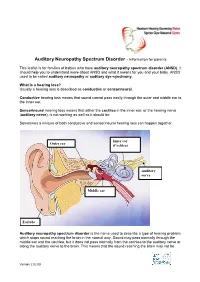
Auditory Neuropathy Spectrum Disorder - Information for Parents
Auditory Neuropathy Spectrum Disorder - Information for parents This leaflet is for families of babies who have auditory neuropathy spectrum disorder (ANSD). It should help you to understand more about ANSD and what it means for you and your baby. ANSD used to be called auditory neuropathy or auditory dys-synchrony. What is a hearing loss? Usually a hearing loss is described as conductive or sensorineural. Conductive hearing loss means that sound cannot pass easily through the outer and middle ear to the inner ear. Sensorineural hearing loss means that either the cochlea in the inner ear, or the hearing nerve (auditory nerve), is not working as well as it should be. Sometimes a mixture of both conductive and sensorineural hearing loss can happen together. Inner ear Outer ear (Cochlea) Auditory nerve Middle ear Earlobe Auditory neuropathy spectrum disorder is the name used to describe a type of hearing problem which stops sound reaching the brain in the normal way. Sound may pass normally through the middle ear and the cochlea, but it does not pass normally from the cochlea to the auditory nerve or along the auditory nerve to the brain. This means that the sound reaching the brain may not be Version 2 02.09 understood and the quality may be poor. The results of different hearing tests will help the audiologist (hearing specialist) decide if your baby has ANSD. Testing for ANSD We can carry out tests that show how well the cochlea and auditory nerve are working, even if your baby is very young. If the tests show the cochlea is working well but the auditory nerve is not working as it should be, then ANSD could be the cause. -

Capturing Activity Along the Auditory Nerve
Into the deep – Capturing activity along the auditory nerve Masterarbeit An der Naturwissenschaftlichen Fakultät der Paris-Lodron-Universität Salzburg im Sommersemester 2018 Eingereicht von Fabian Schmidt Matrikelnummer: 1222361 Gutachter Univ.-Prof. Dr. Nathan Weisz Fachbereich Psychologie INTO THE DEEP 2 Abstract Early auditory evoked potentials occur within the first ten milliseconds after acoustic stimulation. The recording of these potentials usually consists of five to seven vertex positive waves, with Wave I & II being related to activity in the auditory nerve and the cochlear nucleus. The most common used methods of measuring these electrical signals spreading through the auditory pathway are Electrocochleography (ECochG) and the Auditory Brainstem Response (ABR). As the recorded signals are typically weak in amplitude, averaging over a lot of trials, elicited by simple stimuli such as clicks or tone bursts, is required to obtain a reliable response. The large amount of repetitive trials presents a challenge to researchers trying to investigate auditory nerve activity during a more natural stimulation (e.g. listening to running speech). The present study shows, that by combining ECochG, ABR and magnetoencephalography (MEG) using a forward/backward encoding modelling approach, a “pipeline” to the auditory nerve can be built. Results suggest that activity presumably generated by the auditory nerve, can be captured in the MEG. Furthermore, it was shown that early auditory evoked potentials can be reconstructed and used to create a prediction model for the activity along the auditory pathway. This opens the gates to further investigate auditory nerve activity under more natural circumstances (e.g. listening to running speech). keywords: auditory nerve; auditory pathway; cochlear nucleus; early auditory evoked potentials; electrocochleography; magnetoencephalography; auditory brainstem response INTO THE DEEP 3 Zusammenfassung Frühe auditorische evozierte Potentiale ereignen sich in den ersten zehn Millisekunden nach einem akustischen Reiz. -

Simultaneous Extratympanic Electrocochleography and Auditory Brainstem Responses Revisited Carlos Minaya, Samuel R
Audiology Research 2015; volume 5:105 Simultaneous extratympanic electrocochleography and auditory brainstem responses revisited Carlos Minaya, Samuel R. Atcherson Department of Audiology and Speech Pathology, University of Arkansas for Medical Sciences and University of Arkansas at Little Rock, AR, USA Broadband clicks at a fixed level of 85 dB nHL were presented with Abstract alternating polarity at stimulus rates of 9.3, 11.3, and 15.3/s. Different stimulation rates were explored to identify the most efficient rate The purpose of this study was to revisit the two-channel, simultane- without sacrificing time or waveform morphology. Results revealed ous click-evoked extratympanic electrocochleography and auditory larger ECoG AP than ABR Wave I, as expected, and no significant dif- brainstem response (ECoG/ABR) recording technique for clinical use ference across stimulation rate and no interaction effect. in normal hearing participants. Recording the compound action poten- Extratympanic electrode placement takes little additional clinic time tial (AP) of the ECoG simultaneously with ABR may be useful when and may improve the neurodiagnostic utility of the ABR. Wave I of the ABR is small or diminished in patients with sensorineur- al or retrocochlear disorder and minimizes overall test time. In con- trast to some previous studies that used the extratympanic electrode both as non-inverting electrode for the ECoG and inverting electrode Introduction only for ABR, this study maintained separate recording channel montages unique to conventional click-evoked ECoG and ABR recordings. That Electrocochleography (ECoG or ECochG) and auditory brainstem is, the ABR was recorded using a vertical channel (Cz to ipsilateral ear- responses (ABR or BAER) are well-established auditory evoked poten- lobe), while the ECoG with custom extratympanic electrode was tial tests useduse for the assessment of a variety of auditory conditions. -

Use of an Extra-Tympanic Membrane Electrode to Record Cochlear Microphonics with Click, Tone Burst and Chirp Stimuli
Article Use of an Extra-Tympanic Membrane Electrode to Record Cochlear Microphonics with Click, Tone Burst and Chirp Stimuli Laura M. Coraci and Andy J. Beynon * Vestibular & Auditory EP Lab—Department Otorhinolaryngology, Radboud University Medical Center, Ph. Van Leijdenlaan 15, 6525EX Nijmegen, The Netherlands; [email protected] * Correspondence: [email protected] Abstract: This study determined electrocochleography (ECochG) parameter settings to obtain cochlear microphonics (CM) with less invasive flexible extra-tympanic membrane electrodes. In 24 adult normal-hearing subjects, CMs were elicited by presenting click stimuli at 100 dBnHL, tone bursts (2 kHz) and broadband (BB) CE-chirps® LS (Interacoustics, Middelfart, Denmark), both at 80 dBnHL. Different high-pass filters (HPFs) (3.3 Hz and 100 Hz, respectively) were used to investi- gate response quality of the CM. CMs were successfully obtained in 92–100% with click-, 75–83% with 2 kHz tone burst- and 58–63% with CE-chirp®-LS stimuli. Click stimuli elicited significantly larger CM amplitudes compared to 2 kHz tone bursts and BB CE-chirp® LS (Interacoustics, Middelfart, Denmark). No significant differences were found between the two different high-pass filter (HPF) settings. The present study shows that it is possible to obtain clear CMs with the flexible extra- tympanic membrane electrodes using click stimuli. In contrast to 2 kHz tone bursts and CE-chirp® (Interacoustics, Middelfart, Denmark) LS, clicks show a significantly higher success rate and are the preferred stimuli to confirm the presence or absence of CMs. Citation: Coraci, L.M.; Beynon, A.J. Keywords: electrocochleography; cochlear microphonic; tympanic membrane electrode; auditory Use of an Extra-Tympanic Membrane evoked potentials Electrode to Record Cochlear Microphonics with Click, Tone Burst and Chirp Stimuli. -

Starkey Audiology Series
STARKEY AUDIOLOGY SERIES DIAGNOSIS AND MANAGEMENT OF PEDIATRIC AUDITORY NEUROPATHY Deborah Hayes, Ph.D. Chair, Audiology, Speech Pathology and Language Services The Children’s Hospital - Colorado Auditory neuropathy (AN) is a complex auditory resonance imaging (MRI) evidence of auditory nerve disorder, which results in a distinct pattern of abnormality. For an excellent review of auditory audiometric test results and a wide range of neuropathy, see Yvonne Sininger and Arnold Starr symptoms and auditory complaints. The range of (editors): Auditory Neuropathy A New Perspective functional auditory abilities in individuals with AN on Hearing Disorders. is vast and may include normal hearing sensitivity and limited auditory complaints, fluctuating hearing Although the scientific and professional community sensitivity and difficulty understanding speech, have made significant progress in understanding the especially in noise, or profound hearing loss and nature and variable consequences of AN, optimal inability to understand even amplified speech in clinical management options for individuals with quiet despite evidence of normal cochlear outer hair this disorder remain elusive, especially for infants cell function. and very young children. For infants with “typical” sensory hearing loss who are identified through Early reports of auditory neuropathy described newborn hearing screening, excellent and detailed the paradox of individuals with normal pure-tone guidelines for identification, diagnosis, and early audiograms but absent or severely abnormal intervention are widely accepted (Joint Committee auditory brainstem responses (ABR). In some cases, on Infant Hearing, 2007). These guidelines do not individuals demonstrated pure-tone audiograms with address, however, some of the specific challenges mild to moderately-elevated detection thresholds, in providing families with appropriate management absent/abnormal ABRs, and absent middle options for infants with AN. -

Clinical Evaluation of a New Electrocochleography Recording Electrode
Aud Vestib Res (2020);29(2):93-100. DOI: 10.18502/avr.v29i2.2790 RESEARCH ARTICLE Clinical evaluation of a new electrocochleography recording electrode Mostafa Eyvazi1, Akram Pourbakht1, Seyyed Jalal Sameni1*, Mohammad Kamali2,3 1- Department of Audiology, School of Rehabilitation Sciences, Iran University of Medical Sciences, Tehran, Iran 2- Rehabilitation Research Center, Iran University of Medical Sciences, Tehran, Iran 3- Department of Basic Sciences, School of Rehabilitation Sciences, Iran University of Medical Sciences, Tehran, Iran Received: 16 Jan 2020, Revised: 28 Feb 2020, Accepted: 11 Mar 2020, Published: 15 Apr 2020 Abstract Background and Aim: Electrocochleography TIP-trode. The mean SP/AP ratio between two is one of the most practically used tests in app- types of electrodes was statistically significant roaching Meniere's disease (MD). To record reli- differences in normal subjects (p = 0.027) and in able response components, the type of applied MD group (p = 0.009). electrode is of particular importance. The TIP- Conclusion: We demonstrated that the utili- trode is an appropriate electrode due to its user- zation of the Gutter electrode in ECochG assess- friendliness. Gutter electrode can be used in ments was considerably effective and beneficial. more than 100 subject. This study aimed to com- It can significantly reduce expenses and be app- pare the results of the Electrocochleography lied in clinical settings. It’s also recommended (ECochG) test responses using TIP-trode and the that 47.22% mean SP/AP amplitude ratio to be Gutter electrode. considered as upper limit of normality by using Methods: This cross-sectional study was perfor- the Gutter electrode. -

Speech Perception in Individuals with Auditory Neuropathy
Speech Perception in Individuals With Auditory Neuropathy Fan-Gang Zeng Sheng Liu Purpose: Speech perception in participants with auditory neuropathy (AN) was University of California, Irvine systematically studied to answer the following 2 questions: Does noise present a particular problem for people with AN? Can clear speech and cochlear implants alleviate this problem? Method: The researchers evaluated the advantage in intelligibility of clear speech over conversational speech in 13 participants with AN. Of these participants, 7 had received a cochlear implant. Eight sentence-recognition experiments were conducted to examine the clear speech advantage in 2 listening conditions (quiet and noise) using 4 stimulation modes (monaural acoustic, diotic acoustic, monaural electric, and binaurally combined acoustic and electric stimulation). Results: Participants with AN performed more poorly in speech recognition in noise than did the normal-hearing, cochlear-impaired, and cochlear implant controls. A significant clear speech advantage was observed, ranging from 9 to 23 percentage points in intelligibility for all listening conditions and stimulation modes. Electric stimulation via a cochlear implant produced significantly higher intelligibility than acoustic stimulation in both quiet and in noise. Binaural hearing with either diotic acoustic stimulation or combined acoustic and electric stimulation produced significantly higher intelligibility than monaural stimulation in quiet but not in noise. Conclusions: Participants with AN most likely derive the clear speech advantage from enhanced temporal properties in clear speech and improved neural synchrony with electric stimulation. Although the present result supports cochlear implantation as one treatment choice for people with AN, it suggests that the use of innovative hearing aids may be another viable option to improve speech perception in noise. -

A Speed Trap on the Auditory Pathway Investigation of Early Auditory Evoked Brainstem Activity
A Speed Trap on the Auditory Pathway Investigation of early auditory evoked brainstem activity Masterarbeit zur Erlangung des Mastergrades MSc. an der Naturwissenschaftlichen Fakultät der Paris-Lodron Universität Salzburg eingereicht von Florian Geyer Gutachter: Univ.-Prof. Dr. Nathan Weisz Fachbereich: Psychologie Salzburg, November 2018 A Speed Trap on the Auditory Pathway – Investigation of early auditory evoked brainstem activity Abstract In the first 10 ms after acoustic stimulation afferent information “travels” through the auditory pathway. Starting in cochlear nerve these early auditory evoked activity proceeds through the brainstem and parts of the thalamus into the auditory cortex. Early auditory evoked activity usually consists of five to seven positive vertex potentials and can be measured via Electrocochleography or ABR. Those wave peaks can be defined and correlated to their source in the auditory pathway, e.g. wave V is located in the inferior colliculi of the brainstem and is known to elicit a high peak in ABR. Clinicians use early auditory evoked potentials as diagnosis tool for audiological diseases. However, analysis is mostly done visually. This can be challenging. We introduce an approach to get information about the individual wave-region correlation. This has translational potential to clinical applications (e.g. optimization of early auditory evoked activity as a diagnosis tool by facilitating wave detection) and neuroscientific research (e.g. investigation of attentional modulation of early auditory evoked activity). We measured early auditory evoked activity of 18 healthy participants as response to a 30 hz click stimulation at 60 db SPL with magnetic ABR, electrical ABR, ECochG and MEG. We then used Backward Decoding Model to extract a spatial filter from MEG with reference to ABR. -

Diagnosis and Management of Auditory Neuropathy Spectrum
Understanding Auditory Neuropathy: Diagnosis and Management EHDI: Partnering for Progress Raleigh, NC October 26-28, 2011 Patricia Roush, AuD University of North Carolina School of Medicine Chapel Hill, North Carolina, USA University of North Carolina Department of Otolaryngology UNC Hospitals Audiologists: Laura Fleenor McCall, AuD Otolaryngologists: Nissele Franco, AuD Oliver Adunka, MD Corinne Macpherson, AuD Craig Buchman, MD Sarah Martinho, AuD Amelia Drake, MD Patty Reitz, M.S. Austin Rose, MD Jill Ritch, AuD Carlton Zdanski, MD Patricia Roush, AuD Harold Pillsbury, III, MD University of North Carolina Department of Otolaryngology Carolina Children’s Communicative Disorders Program Center for the Acquisition of Spoken Language through Listening Enrichment Audiologists: Debora Hatch, AuD Speech-Language Pathologists: Lisa Park, AuD Hannah Eskridge, AVT Jennifer Woodard, AuD Lillian Henderson, AVT Holly Teagle, AuD Sandra Hancock, AVT Erin Thompson, AVT Maegan Evans, PhD Outline • Overview and Definitions • Protocol for Diagnosis and Management • Case Studies • Implications for Clinical Practice Auditory Neuropathy: A Definition Clinical syndrome characterized by electrophysiological evidence of normal or near normal cochlear function and absent or abnormal auditory pathway transduction Audiologic Findings Ø Normal outer hair cell function as measured by present otoacoustic emissions (OAEs) or the presence of a cochlear microphonic (CM). Ø OAEs may be present initially but disappear over time Ø Abnormal auditory nerve response as observed -

ABR Testing ECOG Testing
Adult & Pediatric Otolaryngology • Head & Neck Surgery • Sinus & Nasal Surgery • Facial Plastic Surgery • Otologic Surgery Auditory Brainstem Response (ABR) and Electrocochleography (ECOG) Testing ABR Testing What is ABR Testing? The Auditory Brainstem Response Test evaluates the auditory nerve pathways from the inner ear to the brainstem. How is the test performed? This diagnostic test obtains auditory information without requiring the active participation of the patient. Very small sensor s (surface electrode tabs) are placed on the forehead and small foam electrodes are also placed just inside the ear canal to present sounds into the ear. The testing begins when click sounds are intro- duced to the ears. The sounds may vary in intensity and tone but should never be uncomfortable. The nerve responses that are picked up by the electrodes and passed on to sophisticated equipment are measured and analyzed by the audiologist. It is painless and non-invasive. The patient is reclined in a comfortable chair. F or best results, the patient is asked to close their eyes, relax, and remain as still as possible. The patient can feel free to fall asleep. Why do I need an ABR? The ABR test can be administered to provide an objective estimate of hearing sensitivity and/or evaluate various neural disorders in the auditory pathways. ABR testing may be performed in cases of asymmetric hearing loss, unilateral tinnitus, sudden hearing loss, or other auditory conditions. What do I need to do before the test? The only requirements for testing are that the patient’s ear canals must be relatively free of wax. -

Utilizing Electrocochleography As a Microphone for Fully Implantable Cochlear Implants William Jason Riggs, Meghan M
www.nature.com/scientificreports OPEN Utilizing Electrocochleography as a Microphone for Fully Implantable Cochlear Implants William Jason Riggs, Meghan M. Hiss, Jefrey Skidmore, Varun V. Varadarajan, Jameson K. Mattingly, Aaron C. Moberly & Oliver F. Adunka* Current cochlear implants (CIs) are semi-implantable devices with an externally worn sound processor that hosts the microphone and sound processor. A fully implantable device, however, would ultimately be desirable as it would be of great beneft to recipients. While some prototypes have been designed and used in a few select cases, one main stumbling block is the sound input. Specifcally, subdermal implantable microphone technology has been poised with physiologic issues such as sound distortion and signal attenuation under the skin. Here we propose an alternative method that utilizes a physiologic response composed of an electrical feld generated by the sensory cells of the inner ear to serve as a sound source microphone for fully implantable hearing technology such as CIs. Electrophysiological results obtained from 14 participants (adult and pediatric) document the feasibility of capturing speech properties within the electrocochleography (ECochG) response. Degradation of formant properties of the stimuli /da/ and /ba/ are evaluated across various degrees of hearing loss. Preliminary results suggest proof-of-concept of using the ECochG response as a microphone is feasible to capture vital properties of speech. However, further signal processing refnement is needed in addition to utilization of an intracochlear recording location to likely improve signal fdelity. To date, it is estimated that as many as 466 million individuals worldwide have hearing loss as defned as an aver- age hearing level of ≥35 dB HL by pure-tone audiometry1. -

An Evaluation of Electrocochleography As a Diagnostic Tool for Ménière's Disease
An Evaluation of Electrocochleography as a Diagnostic Tool for Ménière’s Disease _____________________________________________________________________ A thesis submitted in partial fulfilment of the requirements for the Degree of Master of Audiology in the Department of Communication Disorders at the University of Canterbury By Catherine J. Kalin ____________________________________________________________________ University of Canterbury 2010 ii Acknowledgments _____________________________________________________________________ This master’s thesis could not have been completed without the help and support of many people. I would like to express my gratitude to my two primary supervisors, Dr. Emily Lin and Professor Jeremy Hornibrook, who not only served as my supervisors but also encouraged and challenged me throughout my masters study program. Their passion for research was an inspiration to me, and I would like to thank them both sincerely for the guidance and support they have given me over the past year. I would also like to thank my co-supervisor, Dr Greg O’Beirne, for his help and support with my academic writing. I gratefully acknowledge John Gourley and the audiology staff at Christchurch Hospital who have been involved with electrocochleography recordings over the last 15 years. I would like to thank Angela Harrison at Christchurch Hospital and Glynis Whittaker at the private practice of Mr Hornibrook for the retrieval of the data files. I also wish to thank all the research participants for their contributions in this study. I sincerely thank you all for allowing me to have access to your medical files. Finally, I would like to acknowledge the loving support of my friends and family. To my fellow postgraduate students, I am most grateful for your ongoing support, encouragement and company.