Identification and Management of Infants and Young Children with Consensus
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Pathophysiological Mechanisms and Functional Hearing Consequences of Auditory Neuropathy
doi:10.1093/brain/awv270 BRAIN 2015: 138; 3141–3158 | 3141 REVIEW ARTICLE Pathophysiological mechanisms and functional hearing consequences of auditory neuropathy Gary Rance1 and Arnold Starr2 The effects of inner ear abnormality on audibility have been explored since the early 20th century when sound detection measures were first used to define and quantify ‘hearing loss’. The development in the 1970s of objective measures of cochlear hair cell Downloaded from function (cochlear microphonics, otoacoustic emissions, summating potentials) and auditory nerve/brainstem activity (auditory brainstem responses) have made it possible to distinguish both synaptic and auditory nerve disorders from sensory receptor loss. This distinction is critically important when considering aetiology and management. In this review we address the clinical and pathophysiological features of auditory neuropathy that distinguish site(s) of dysfunction. We describe the diagnostic criteria for: (i) presynaptic disorders affecting inner hair cells and ribbon synapses; (ii) postsynaptic disorders affecting unmyelinated auditory http://brain.oxfordjournals.org/ nerve dendrites; (iii) postsynaptic disorders affecting auditory ganglion cells and their myelinated axons and dendrites; and (iv) central neural pathway disorders affecting the auditory brainstem. We review data and principles to identify treatment options for affected patients and explore their benefits as a function of site of lesion. 1 Department of Audiology and Speech Pathology, The University of Melbourne, -

A Guide to Otoacoustic Emissions Contents
Science made smarter A Guide to Otoacoustic Emissions Contents What are OAEs? ................................................................................ 4 Stimulus tolerance............................................................................................................................................................22 Filtering (TEOAE) ................................................................................................................................................................22 What are Otoacoustic Emissions (OAEs)? ........................................................... 4 Noise rejection level ......................................................................................................................................................23 Types of OAEs ................................................................................................................ 5 Measuring at ambient or tympanic peak pressure (pressurized OAE) ...........................................23 DPOAEs ............................................................................................................................. 6 OAE detection/stopping criteria ............................................................................................................................25 TEOAEs .............................................................................................................................. 7 Screening vs diagnostic OAE ........................................................8 Before and -

Audiology 101: an Introduction to Audiology for Nonaudiologists Terry Foust, Aud, FAAA, CC-SLP/A; & Jeff Hoffman, MS, CCC-A
NATIONALA RESOURCE CENTER GUIDE FOR FOR EARLY HEARING HEARING ASSESSMENT DETECTION & & MANAGEMENT INTERVENTION Chapter 5 Audiology 101: An Introduction to Audiology for Nonaudiologists Terry Foust, AuD, FAAA, CC-SLP/A; & Jeff Hoffman, MS, CCC-A Parents of young Introduction What is an audiologist? children who are arents of young children who are An audiologist is a specialist in hearing identified as deaf or hard identified as deaf or hard of hearing and balance who typically works in of hearing (DHH) are P(DHH) are suddenly thrust into a either a medical, private practice, or an suddenly thrust into a world of new concepts and a bewildering educational setting. The primary roles of world of new concepts array of terms. What’s a decibel or hertz? an audiologist include the identification and a bewildering array What does sensorineural mean? Is a and assessment of hearing and balance moderate hearing loss one to be concerned problems, the habilitation or rehabilitation of terms. about, since it’s only moderate? What’s of hearing and balance problems, and the a tympanogram or a cochlear implant? prevention of hearing loss. When working These are just a few of the many questions with infants and young children, the that a parent whose child has been primary focus of audiology is hearing. identified as DHH may have. In addition to parents, questions also arise from Audiologists are licensed by the state in professionals and paraprofessionals who which they practice and may be members work in the field of early hearing detection of the American Speech-Language- and intervention (EHDI) and are not Hearing Association (ASHA), American audiologists. -
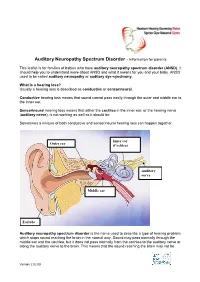
Auditory Neuropathy Spectrum Disorder - Information for Parents
Auditory Neuropathy Spectrum Disorder - Information for parents This leaflet is for families of babies who have auditory neuropathy spectrum disorder (ANSD). It should help you to understand more about ANSD and what it means for you and your baby. ANSD used to be called auditory neuropathy or auditory dys-synchrony. What is a hearing loss? Usually a hearing loss is described as conductive or sensorineural. Conductive hearing loss means that sound cannot pass easily through the outer and middle ear to the inner ear. Sensorineural hearing loss means that either the cochlea in the inner ear, or the hearing nerve (auditory nerve), is not working as well as it should be. Sometimes a mixture of both conductive and sensorineural hearing loss can happen together. Inner ear Outer ear (Cochlea) Auditory nerve Middle ear Earlobe Auditory neuropathy spectrum disorder is the name used to describe a type of hearing problem which stops sound reaching the brain in the normal way. Sound may pass normally through the middle ear and the cochlea, but it does not pass normally from the cochlea to the auditory nerve or along the auditory nerve to the brain. This means that the sound reaching the brain may not be Version 2 02.09 understood and the quality may be poor. The results of different hearing tests will help the audiologist (hearing specialist) decide if your baby has ANSD. Testing for ANSD We can carry out tests that show how well the cochlea and auditory nerve are working, even if your baby is very young. If the tests show the cochlea is working well but the auditory nerve is not working as it should be, then ANSD could be the cause. -

Non-Commercial Use Only
Audiology Research 2013; volume 3:e6 Comparison of cervical and ocular vestibular evoked myogenic potentials in dancers and non-dancers Sujeet Kumar Sinha, Vaishnavi Bohra, Himanshu Kumar Sanju Department of Audiology, All India Institute of Speech and Hearing, India Abstract Introduction The objective of the study was to assess the sacculocollic and otolith In recent years, cervical vestibular evoked myogenic potentials ocular pathway function using cervical vestibular evoked myogenic (cVEMP) have been utilized for the diagnosis of various disorders such potentials (cVEMP) and ocular vestibular myogenic potentials as, Meniere’s disease,1,2 acoustic neuroma,2-5 superior canal dehis- (oVEMP) in dancers and non dancers. Total 16 subjects participated in cence,6 vestibular neuritis,7 benign paroxysmal positional vertigo,8 the study. Out of 16 participants, 8 were trained in Indian classical noise induced hearing loss,9,10 auditory neuropathy/audiovestibular form of dance (dancers) and other 8 participants who were not trained neuropathy,10,11 as well as other disorders such as cerebellopontine in any dance form (non dancers). cVEMP and oVEMP responses were angle tumor,12 and multiple sclerosis.2 Similarly, ocular vestibular recorded for all the subjects. Non Parametric Mann-Whitney U test evoked myogenic potentials (oVEMP) also have been utilised in diag- revealed no significant difference between dancers and non dancers 13 for the latency and amplitude parameter for cVEMP and oVEMP, i.e. nosing superior semicircular canal dehiscence syndrome, internu- 14 P13, N23 latency and P13-N23 complex amplitude and N10, P14 laten- clearophthalmoplegia, to differentiateonly between cerebellar and brain- cy, N10-P14 complex amplitude respectively. -

Cranial Nerve VIII
Cranial Nerve VIII Color Code Important (The Vestibulo-Cochlear Nerve) Doctors Notes Notes/Extra explanation Please view our Editing File before studying this lecture to check for any changes. Objectives At the end of the lecture, the students should be able to: ✓ List the nuclei related to vestibular and cochlear nerves in the brain stem. ✓ Describe the type and site of each nucleus. ✓ Describe the vestibular pathways and its main connections. ✓ Describe the auditory pathway and its main connections. Due to the difference of arrangement of the lecture between the girls and boys slides we will stick to the girls slides then summarize the pathway according to the boys slides. Ponto-medullary Sulcus (cerebello- pontine angle) Recall: both cranial nerves 8 and 7 emerge from the ventral surface of the brainstem at the ponto- medullary sulcus (cerebello-pontine angle) Brain – Ventral Surface Vestibulo-Cochlear (VIII) 8th Cranial Nerve o Type: Special sensory (SSA) o Conveys impulses from inner ear to nervous system. o Components: • Vestibular part: conveys impulses associated with body posture ,balance and coordination of head & eye movements. • Cochlear part: conveys impulses associated with hearing. o Vestibular & cochlear parts leave the ventral surface* of brain stem through the pontomedullary sulcus ‘at cerebellopontine angle*’ (lateral to facial nerve), run laterally in posterior cranial fossa and enter the internal acoustic meatus along with 7th (facial) nerve. *see the previous slide Auditory Pathway Only on the girls’ slides 04:14 Characteristics: o It is a multisynaptic pathway o There are several locations between medulla and the thalamus where axons may synapse and not all the fibers behave in the same manner. -

Audiology and Hearing Aid Services
For more information, call the Hearing Aid Services office nearest you: Comprehensive hearing aid related services Barbourville Bowling Green are available to children diagnosed with (800) 348-4279 (800) 843-5877 permanent childhood hearing loss (PCHL). (606) 546-5109 (270) 746-7816 Elizabethtown Hazard Who should be referred to the OCSHCN (800) 995-6982 (800) 378-3357 Hearing Aid Services program? (270) 766-5370 (606) 435-6167 Children who are in need of new or Lexington Louisville replacement hearing aids and want to (800) 817-3874 (800) 232-1160 receive hearing aids and related services (859) 252-3170 (502) 429-4430 through a OCSHCN audiologist and wish to Morehead Owensboro receive Otology care outside of the (800) 928-3049 (877) 687-7038 clinical Otology program. (606) 783-8610 (270) 687-7038 What audiology services are available Paducah Prestonsburg (800) 443-3651 (800) 594-7058 through the OCSHCN Hearing Aid Services (270) 443-3651 (606) 889-1761 program? Licensed, certified audiologists conduct Somerset (800) 525-4279 periodic comprehensive hearing evaluations, (606) 677-4120 hearing aid checks, hearing aid repairs and Audiology and hearing aid evaluations according to ASHA best practices guidelines. Comprehensive Kentucky Cabinet for Health and Family Services Hearing Aid Services Office for Children with Special Health Care Needs reports are provided to the managing 310 Whittington Parkway, Suite 200, Louisville, KY 40222 otolaryngologist on an on-going basis; Phone: (502) 429-4430 or (800) 232-1160 FAX: (502) 429-4489 additional follow up testing will be http://chfs.ky.gov/agencies/ccshcn Information for Parents and Equal Opportunity Employer M/D/F completed at physician request. -

Audiometry Procedures Manual
Audiometry Procedures Manual January 2008 TABLE OF CONTENTS Chapter Page 1 INTRODUCTION ........................................................................................... 1-1 1.1 History and Overview of Hearing Examinations in NHANES........... 1-1 1.2 Basic Principles of Sound................................................................... 1-5 1.3 Basic Principles of Audition............................................................... 1-8 1.4 Basic Principles of Hearing Loss........................................................ 1-10 2 EQUIPMENT .................................................................................................. 2-1 2.1 Description of Exam Room in MEC................................................... 2-1 2.2 Description of Equipment and Supplies ............................................. 2-2 2.2.1 Otoscope.............................................................................. 2-9 2.2.2 Tympanometer .................................................................... 2-9 2.2.3 Audiometer.......................................................................... 2-9 2.2.4 Bioacoustic Simulator ......................................................... 2-10 2.2.5 Sound Level Meter and Accessories ................................... 2-10 2.2.6 Inventory Procedures .......................................................... 2-11 2.3 Start of Stand Procedures.................................................................... 2-11 2.3.1 Room Setup........................................................................ -

A Rare Case of Hearing Impairment Due to Cerebello-Pontine Angle Lesion: Trigeminal Schwannoma
J Int Adv Otol 2015; 11(2): 170-2 • DOI: 10.5152/iao.2015.252 Case Report A Rare Case of Hearing Impairment due to Cerebello-Pontine Angle Lesion: Trigeminal Schwannoma Purushothaman Ganesan, Priya Sankaran, Purushothaman Pavanjur Kothandaraman SRM Medical College Hospital and Research Centre, Otorhinolaryngology, Audiology and Speech Language Pathology, Chennai, Tamilnadu, India Schwannoma of the trigeminal nerve is a rare condition. Even rarer is hearing loss occurring as a result of this lesion. The aim of this study is to highlight this rare cause of hearing impairment. Here we report the clinical features and findings of the imaging and audiological investigations of a case of trigeminal schwannoma diagnosed at our institution. Our patient presented with headache, giddiness, tinnitus, left-sided facial weakness, left-sided hearing loss, right-sided hemiplegia, and unintelligible speech. Radiological studies revealed a large well-defined mass lesion in the left cerebellopontine angle with a significant mass effect on posterior fossa structures, suggestive of trigeminal nerve tumor. Audio-vestibular assess- ment was done with pure tone audiometry, impedance audiometry, otoacoustic emission, brainstem-evoked response audiometry, and electronys- tagmography, which pointed toward a retrocochlear pathology for hearing loss and imbalance. KEYWORDS: Cerebellopontine angle tumor, vestibulocochlear nerve bundle, trigeminal schwannoma, electronystagmography INTRODUCTION Schwannomas, which usually exhibit benign behavior, are tumors arising from Schwann cells in the axon myelin sheaths [1]. Schwan- noma of the trigeminal nerve comprises only 0.2% to 0.4% of all intracranial tumors and primarily arises in the Gasserian ganglion [2]. They are relatively rare and less common than vestibular schwannoma [3]. They account for 0.07%–0.36% of all intracranial tumors and 0.8%–8% of intracranial schwannomas [4, 5]. -

Benzodiazepines Alter Cochleo-Cochlear Loop in Humans
Hearing Research 121 (1998) 71^76 Benzodiazepines alter cochleo-cochlear loop in humans N. Morand a, E. Veuillet a;*, M.C. Gagnieu b, P. Lemoine c, L. Collet a a Laboratoire `Neurosciences et Systeémes Sensoriels', 3 place d'Arsonval, Pavillon U, Hoêpital Edouard Herriot, 69003 Lyon, France b Laboratoire de Pharmacologie, Hoêpital Edouard Herriot, Lyon, France c Uniteè Clinique de Psychiatrie Biologique, Le Vinatier, Bron, France Received 26 November 1997; revised 26 March 1998; accepted 7 April 1998 Abstract By using otoacoustic emission, we looked for change in outer hair cell (OHC) motile activity and medial olivocochlear (MOC) system inhibition due to benzodiazepine administration, a drug that is known to produce a pharmacological effect by interacting with GABAergic inhibitory neurotransmission. No effect was observed on OHC motile activity, in contrast benzodiazepines decreased MOC system effectiveness suggesting the existence of GABAergic fibers projecting onto the MOC system. z 1998 Elsevier Science B.V. All rights reserved. Key words: Gamma-aminobutyric acid; Outer hair cell; Olivocochlear e¡erent system; Otoacoustic emission 1. Introduction OHCs, principally acetylcholine (Ach) and the inhibi- tory neurotransmitter Q-aminobutyric acid (GABA) (for The organ of Corti is innervated by the olivocochlear review see Eybalin, 1993). Concerning GABA, several e¡erent system (Rasmussen, 1946), in which two sub- studies seem to prove the presence of GABAergic syn- components have been clearly distinguished (Warr, apses in the cochlea. The presence of GABA and glu- 1992; Warr and Guinan, 1979). The lateral olivo- tamic acid decarboxylase in ¢bers and e¡erent endings cochlear (LOC) system originates from lateral superior synapsing onto OHC bodies has been revealed by im- olivary neurons making synaptic contact with the a¡er- munocytochemistry data (Fex and Altschuler, 1984; ent inner radial ¢bers of the inner hair cell system. -

Cochlear Implant Guide
Cochlear Implant Guide Ear & Hearing Center at Texas Children’s Hospital® Dear Families, Welcome to the Cochlear Implant Program at Texas Children’s Hospital! Our goal is to provide comprehensive, specialized care for children with severe hearing loss, and help them achieve the best possible communication outcomes. Since our program started in 2001, we have performed more than 700 implants. Your child may be a candidate for a cochlear implant in one or both ears. Cochlear implants, or CIs, are innovative devices which can directly stimulate the auditory nerve and provide access to sound in children with severe or profound hearing loss. The decision about whether to recommend a CI is highly individualized, and requires the input of specialists in multiple areas, including Otolaryngology, Audiology, Speech Pathology, and Neuropsychology, among others. These specialists make up the members of the Cochlear Implant Team. They work in close collaboration to determine who is a candidate for a CI, perform the implantation surgery, and help patients through the rehabilitation afterwards. This guide will help you understand the entire process, who may be considered a CI candidate, the basics of how the device works, how the surgery is done, and the necessary rehabilitation to achieve a successful outcome. Thank you for the opportunity to help your family through this journey. All of our team members are dedicated to helping children with hearing loss achieve their full potential. If you have any questions or concerns, please do not hesitate to contact us. Sincerely, The Cochlear Implant Team at Texas Children’s Hospital Table of Contents 1. -

Anatomy of the Ear ANATOMY & Glossary of Terms
Anatomy of the Ear ANATOMY & Glossary of Terms By Vestibular Disorders Association HEARING & ANATOMY BALANCE The human inner ear contains two divisions: the hearing (auditory) The human ear contains component—the cochlea, and a balance (vestibular) component—the two components: auditory peripheral vestibular system. Peripheral in this context refers to (cochlea) & balance a system that is outside of the central nervous system (brain and (vestibular). brainstem). The peripheral vestibular system sends information to the brain and brainstem. The vestibular system in each ear consists of a complex series of passageways and chambers within the bony skull. Within these ARTICLE passageways are tubes (semicircular canals), and sacs (a utricle and saccule), filled with a fluid called endolymph. Around the outside of the tubes and sacs is a different fluid called perilymph. Both of these fluids are of precise chemical compositions, and they are different. The mechanism that regulates the amount and composition of these fluids is 04 important to the proper functioning of the inner ear. Each of the semicircular canals is located in a different spatial plane. They are located at right angles to each other and to those in the ear on the opposite side of the head. At the base of each canal is a swelling DID THIS ARTICLE (ampulla) and within each ampulla is a sensory receptor (cupula). HELP YOU? MOVEMENT AND BALANCE SUPPORT VEDA @ VESTIBULAR.ORG With head movement in the plane or angle in which a canal is positioned, the endo-lymphatic fluid within that canal, because of inertia, lags behind. When this fluid lags behind, the sensory receptor within the canal is bent.