Signals Emanating from Leukocyte-Endothelium Interactions During Inflammation Recibido El 28 De Mayo De 2008
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Paxillin Binding to the Cytoplasmic Domain of CD103 Promotes Cell Adhesion and Effector
Author Manuscript Published OnlineFirst on October 11, 2017; DOI: 10.1158/0008-5472.CAN-17-1487 Author manuscripts have been peer reviewed and accepted for publication but have not yet been edited. Paxillin binding to the cytoplasmic domain of CD103 promotes cell adhesion and effector functions for CD8+ resident memory T cells in tumors Ludiane Gauthier1, Stéphanie Corgnac1, Marie Boutet1, Gwendoline Gros1, Pierre Validire2, Georges Bismuth3 and Fathia Mami-Chouaib1 1 INSERM UMR 1186, Integrative Tumor Immunology and Genetic Oncology, Gustave Roussy, EPHE, Fac. de médecine - Univ. Paris-Sud, Université Paris-Saclay, 94805, Villejuif, France 2 Institut Mutualiste Montsouris, Service d’Anatomie pathologique, 75014 Paris, France. 3 INSERM U1016, CNRS UMR8104, Université Paris Descartes, Institut Cochin, 75014 Paris. S Corgnac, M Boutet and G Gros contributed equally to this work. M Boutet current address: Department of Microbiology and Immunology Albert Einstein College of Medecine, NY 10461 USA. Corresponding author: Fathia Mami-Chouaib, INSERM UMR 1186, Gustave Roussy. 39, rue Camille Desmoulins, F-94805 Villejuif. Phone: +33 1 42 11 49 65, Fax: +33 1 42 11 52 88, e-mail: [email protected] and [email protected] Running title: CD103 signaling in human TRM cells Key words: TRM cells, CD103 integrin, T-cell function and signaling, paxillin. Abbreviations: IS: immune synapse; LFA: leukocyte function-associated antigen; FI: fluorescence intensity; mAb: monoclonal antibody; phospho: phosphorylated; Pyk2: proline- rich tyrosine kinase-2; NSCLC: non-small-cell lung carcinoma; r: recombinant; sh-pxn: shorthairpin RNA-paxillin; TCR: T-cell receptor; TIL: tumor-infiltrating lymphocyte; TRM: tissue-resident memory T. -

Human Integrin Alpha L Gene Cdna Clone Plasmid
Human Integrin alpha L Gene cDNA clone plasmid Catalog Number: HG10812-M General Information Plasmid Resuspension protocol Gene : integrin, alpha L (antigen CD11A (p180), lymphocyte function-associated antigen 1; 1.Centrifuge at 5,000×g for 5 min. alpha polypeptide) 2.Carefully open the tube and add 100 l of sterile water to dissolve the DNA. Official Symbol : ITGAL 3.Close the tube and incubate for 10 minutes at room temperature. 4.Briefly vortex the tube and then do a quick spin to concentrate Synonym : CD11A, LFA-1, LFA1A, ITGAL the liquid at the bottom. Speed is less than 5000×g. 5.Store the plasmid at -20 ℃. Source : Human The plasmid is ready for: cDNA Size: 3513bp • Restriction enzyme digestion • PCR amplification RefSeq : NM_002209.2 • E. coli transformation • DNA sequencing Plasmid: pMD-ITGAL E.coli strains for transformation (recommended Description but not limited) Lot : Please refer to the label on the tube Most commercially available competent cells are appropriate for Sequence Description : the plasmid, e.g. TOP10, DH5α and TOP10F´. Identical with the Gene Bank Ref. ID sequence except for the point mutation 2928 A/G not causing the amino acid variation. Vector : pMD18-T Simple Shipping carrier : Each tube contains approximately 10 μg of lyophilized plasmid. Storage : The lyophilized plasmid can be stored at ambient temperature for three months. Quality control : The plasmid is confirmed by full-length sequencing with primers in the sequencing primer list. Sequencing primer list : M13-47 : 5’ GCCAGGGTTTTCCCAGTCACGAC 3’ RV-M : 5’ GAGCGGATAACAATTTCACACAGG 3’ Other M13 primers can also be used as sequencing primers. -

Supplementary Table 1: Adhesion Genes Data Set
Supplementary Table 1: Adhesion genes data set PROBE Entrez Gene ID Celera Gene ID Gene_Symbol Gene_Name 160832 1 hCG201364.3 A1BG alpha-1-B glycoprotein 223658 1 hCG201364.3 A1BG alpha-1-B glycoprotein 212988 102 hCG40040.3 ADAM10 ADAM metallopeptidase domain 10 133411 4185 hCG28232.2 ADAM11 ADAM metallopeptidase domain 11 110695 8038 hCG40937.4 ADAM12 ADAM metallopeptidase domain 12 (meltrin alpha) 195222 8038 hCG40937.4 ADAM12 ADAM metallopeptidase domain 12 (meltrin alpha) 165344 8751 hCG20021.3 ADAM15 ADAM metallopeptidase domain 15 (metargidin) 189065 6868 null ADAM17 ADAM metallopeptidase domain 17 (tumor necrosis factor, alpha, converting enzyme) 108119 8728 hCG15398.4 ADAM19 ADAM metallopeptidase domain 19 (meltrin beta) 117763 8748 hCG20675.3 ADAM20 ADAM metallopeptidase domain 20 126448 8747 hCG1785634.2 ADAM21 ADAM metallopeptidase domain 21 208981 8747 hCG1785634.2|hCG2042897 ADAM21 ADAM metallopeptidase domain 21 180903 53616 hCG17212.4 ADAM22 ADAM metallopeptidase domain 22 177272 8745 hCG1811623.1 ADAM23 ADAM metallopeptidase domain 23 102384 10863 hCG1818505.1 ADAM28 ADAM metallopeptidase domain 28 119968 11086 hCG1786734.2 ADAM29 ADAM metallopeptidase domain 29 205542 11085 hCG1997196.1 ADAM30 ADAM metallopeptidase domain 30 148417 80332 hCG39255.4 ADAM33 ADAM metallopeptidase domain 33 140492 8756 hCG1789002.2 ADAM7 ADAM metallopeptidase domain 7 122603 101 hCG1816947.1 ADAM8 ADAM metallopeptidase domain 8 183965 8754 hCG1996391 ADAM9 ADAM metallopeptidase domain 9 (meltrin gamma) 129974 27299 hCG15447.3 ADAMDEC1 ADAM-like, -

Human ITGAL Peptide (DAG-P0325) This Product Is for Research Use Only and Is Not Intended for Diagnostic Use
Human ITGAL peptide (DAG-P0325) This product is for research use only and is not intended for diagnostic use. PRODUCT INFORMATION Antigen Description ITGAL encodes the integrin alpha L chain. Integrins are heterodimeric integral membrane proteins composed of an alpha chain and a beta chain. This I-domain containing alpha integrin combines with the beta 2 chain (ITGB2) to form the integrin lymphocyte function-associated antigen-1 (LFA-1), which is expressed on all leukocytes. LFA-1 plays a central role in leukocyte intercellular adhesion through interactions with its ligands, ICAMs 1-3 (intercellular adhesion molecules 1 through 3), and also functions in lymphocyte costimulatory signaling. Two transcript variants encoding different isoforms have been found for this gene. [provided by RefSeq, Jul 2008] Specificity Leukocytes. Nature Synthetic Expression System N/A Purity 70 - 90% by HPLC. Conjugate Unconjugated Sequence Similarities Belongs to the integrin alpha chain family.Contains 7 FG-GAP repeats.Contains 1 VWFA domain. Cellular Localization Membrane. Procedure None Format Liquid Preservative None Storage Shipped at 4°C. Upon delivery aliquot and store at -20°C or -80°C. Avoid repeated freeze / thaw cycles. Information available upon request. ANTIGEN GENE INFORMATION Gene Name ITGAL integrin, alpha L (antigen CD11A (p180), lymphocyte function-associated antigen 1; alpha polypeptide) [ Homo sapiens (human) ] 45-1 Ramsey Road, Shirley, NY 11967, USA Email: [email protected] Tel: 1-631-624-4882 Fax: 1-631-938-8221 1 © Creative -
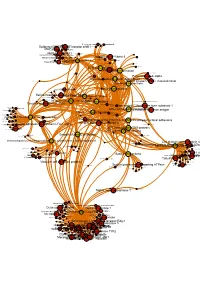
Brcaallwithlabelsintegrina1b1
Receptor-type tyrosine-proteinMuscle- skeletal receptor phosphatase tyrosine protein kinase S Dual specificity mitogen-activated protein kinase kinase 1 Plasminogen activator inhibitor-1 Epidermal growth factor receptor erbB1 Beta amyloid A4 protein MAPDual specificity Epidermalkinase mitogen-activated growth factorCasein proteinreceptor ERK2 kinase and ErbB2kinase; II (HER1 alphaMEK1/2 and (prime) HER2) Casein kinase II alpha Integrin alpha-IIb MAPCasein kinase kinase II beta ERK1 Dual specificity mitogen-activated protein kinase kinase 2 Lysyl oxidase Integrin alpha-IIb/beta-3 Furin Mitogen-activatedSerine/threonine-protein kinase RAF and Dual specificityEpidermal protein mitogen-activated growth proteinkinase; factor kinase kinase receptor 1 (Raf/MEK)ERK1/ERK2 Integrin alpha-V/beta-3 Integrin alpha-V/beta-3 and alpha-IIb/beta 3 Casein kinase II Signal transduction by L1 Integrin alpha-V/beta-6 Casein kinase II alpha/beta Vitronectin receptor alpha Bone morphogenetic protein 2 ECM proteoglycans Bone morphogenetic protein 4 VitronectinElastic fibre formation Peripheral plasma membrane protein CASK Neuropilin-1 Integrin alpha-V/beta-5 Protein kinaseProtein kinase C (PKC) alpha Integrin alpha-2/beta-3 Molecules associated with elastic fibres PKC alpha and beta-2 MER intracellular domain/EGFR extracellular domain chimera Fibronectin receptor alpha Protein kinase C- PKC; classical/novel Syndecan interactions Integrin alpha-2 von Willebrand factorIntegrin alpha-10 Laminin interactions Integrin alpha-11 Fibrinogen beta chain Serine/threonine-proteinVascular -

ICAM-1 (Phospho Tyr512) Polyclonal Antibody
ICAM-1 Monoclonal Antibody Catalog No : YM1051 Reactivity : Human Applications : WB,IF/ICC Gene Name : ICAM1 Protein Name : Intercellular adhesion molecule 1 Human Gene Id : 3383 Human Swiss Prot P05362 No : Mouse Swiss Prot P13597 No : Immunogen : Purified recombinant human ICAM-1 (N-terminus) protein fragments expressed in E.coli. Specificity : ICAM-1 Monoclonal Antibody detects endogenous levels of ICAM-1 protein. Formulation : Purified mouse monoclonal in buffer containing 0.1M Tris-Glycine (pH 7.4, 150 mM NaCl) with 0.2% sodium azide, 50% glycerol. Source : Mouse Dilution : Western Blot: 1/1000 - 1/2000. Immunofluorescence: 1/100 - 1/500. Not yet tested in other applications. Purification : Affinity purification Concentration : 1 mg/ml Storage Stability : -20°C/1 year Cell Pathway : Cell adhesion molecules (CAMs),Natural killer cell mediated cytotoxicity,Leukocyte transendothelial migration,Viral myocarditis, 1 / 2 Background : intercellular adhesion molecule 1(ICAM1) Homo sapiens This gene encodes a cell surface glycoprotein which is typically expressed on endothelial cells and cells of the immune system. It binds to integrins of type CD11a / CD18, or CD11b / CD18 and is also exploited by Rhinovirus as a receptor. [provided by RefSeq, Jul 2008], Function : function:ICAM proteins are ligands for the leukocyte adhesion protein LFA-1 (integrin alpha-L/beta-2). During leukocyte trans-endothelial migration, ICAM1 engagement promotes the assembly of endothelial apical cups through SGEF and RHOG activation. In case of rhinovirus infection acts as a cellular receptor for the virus.,online information:ICAM-1,online information:Icosahedral capsid structure,online information:Intercellular adhesion molecule entry,polymorphism:Homozygotes with ICAM1-Kalifi Met-56 seem to have an increased risk for cerebral malaria.,PTM:Monoubiquitinated, which is promoted by MARCH9 and leads to endocytosis.,similarity:Belongs to the immunoglobulin superfamily. -

CD11/CD18 (24) FITC FITC Labeled Mouse Monoclonal Antibody
Product Data Sheet IMQ-84601_2015.09-v1 CD11/CD18 (24) FITC FITC Labeled Mouse Monoclonal Antibody Catalog Number: IMQ-84601 Size: 50 µl (1 mg/ml) Class: Monoclonal Type: Antibody Clone: 24 Host / Isotype: Mouse / IgG1 Immunogen: Fibronectin-purified human monocytes Myeloma / fusion Cells from immunized Balb/c mice were fused with the Sp2/0-Ag.14 myeloma partners: cell line Species Reactivity: Human Specificity: This antibody recognises integrin alpha L,M,X/Beta 2 (CD11/CD18). Integrins are heterodimeric cell surface receptors composed of alpha and beta subunits which mediate cell-cell and cell-extracellular matrix attachments. Integrin beta2 (CD18) associates with integrin alpha L (CD11a) to form the leukocyte function- associated antigen-1 (LFA-1) with integrin alpha M (CD11b) to form Complement Receptor 3 (CR3) and with integrin alpha X (CD11c) to form Complement Receptor 4 (CR4). Each integrin receptor can bind various Intercellular adhesion molecules (ICAMs). Aberrant integrin expression has been found in many epithelial tumours. Changes in integrin expression have been shown to be important for the growth and early metastatic capacity of melanoma cells. Purification: Purified on protein A from tissue culture supernatant. Format: Purified IgG conjugated to Fluoroscein using Innova Biosciences Lightning- Link®, supplied in Phosphate buffered saline (PBS) containing 0.09% Sodium azide Applications: Flow Cytometry, Immunocytochemistry, Immunofluorescence Dilutions: Optimal antibody dilution should be determined by titration Storage: Store stock solution of the antibody at 2-8 °C in the dark. Do not freeze. References: Hogg N, et al. An anti-human monocyte/macrophage monoclonal antibody, reacting most strongly with macrophages in lymphoid tissue. -

PDF Download
Integrin β2 Polyclonal Antibody Catalog No : YT2369 Reactivity : Human,Mouse,Rat Applications : IF/ICC,ELISA Gene Name : ITGB2 Protein Name : Integrin beta-2 Human Gene Id : 3689 Human Swiss Prot P05107 No : Mouse Gene Id : 16414 Mouse Swiss Prot P11835 No : Immunogen : The antiserum was produced against synthesized peptide derived from human CD18/ITGB2. AA range:720-769 Specificity : Integrin β2 Polyclonal Antibody detects endogenous levels of Integrin β2 protein. Formulation : Liquid in PBS containing 50% glycerol, 0.5% BSA and 0.02% sodium azide. Source : Rabbit Dilution : Immunofluorescence: 1/200 - 1/1000. ELISA: 1/5000. Not yet tested in other applications. Purification : The antibody was affinity-purified from rabbit antiserum by affinity- chromatography using epitope-specific immunogen. Concentration : 1 mg/ml Storage Stability : -20°C/1 year Molecularweight : 84782 1 / 2 Cell Pathway : Cell adhesion molecules (CAMs),Natural killer cell mediated cytotoxicity,Leukocyte transendothelial migration,Regulates Actin and Cytoskeleton,Viral myocarditis, Background : integrin subunit beta 2(ITGB2) Homo sapiens This gene encodes an integrin beta chain, which combines with multiple different alpha chains to form different integrin heterodimers. Integrins are integral cell-surface proteins that participate in cell adhesion as well as cell-surface mediated signalling. The encoded protein plays an important role in immune response and defects in this gene cause leukocyte adhesion deficiency. Alternative splicing results in multiple transcript variants. [provided by RefSeq, Dec 2014], Function : disease:Defects in ITGB2 are the cause of leukocyte adhesion deficiency type I (LAD1) [MIM:116920]. LAD1 patients have recurrent bacterial infections and their leukocytes are deficient in a wide range of adhesion-dependent functions.,function:Integrin alpha-L/beta-2 is a receptor for ICAM1, ICAM2, ICAM3 and ICAM4. -

Anti-CD54 / ICAM1 Antibody (ARG40213)
Product datasheet [email protected] ARG40213 Package: 100 μl anti-CD54 / ICAM1 antibody Store at: -20°C Summary Product Description Rabbit Polyclonal antibody recognizes CD54 / ICAM1 Tested Reactivity Hu, Ms, Rat Tested Application ICC/IF, WB Host Rabbit Clonality Polyclonal Isotype IgG Target Name CD54 / ICAM1 Antigen Species Human Immunogen Recombinant protein of Human CD54 / ICAM1. (NP_000192.2) Conjugation Un-conjugated Alternate Names CD54; CD antigen CD54; BB2; P3.58; Intercellular adhesion molecule 1; Major group rhinovirus receptor; ICAM-1 Application Instructions Application table Application Dilution ICC/IF 1:50 - 1:200 WB 1:500 - 1:2000 Application Note * The dilutions indicate recommended starting dilutions and the optimal dilutions or concentrations should be determined by the scientist. Positive Control Rat lung, Mouse lung and Raji Calculated Mw 58 kDa Properties Form Liquid Purification Affinity purified. Buffer PBS (pH 7.3), 0.02% Sodium azide and 50% Glycerol. Preservative 0.02% Sodium azide Stabilizer 50% Glycerol Storage instruction For continuous use, store undiluted antibody at 2-8°C for up to a week. For long-term storage, aliquot and store at -20°C. Storage in frost free freezers is not recommended. Avoid repeated freeze/thaw cycles. Suggest spin the vial prior to opening. The antibody solution should be gently mixed before use. Note For laboratory research only, not for drug, diagnostic or other use. www.arigobio.com 1/2 Bioinformation Gene Symbol ICAM1 Gene Full Name intercellular adhesion molecule 1 Background This gene encodes a cell surface glycoprotein which is typically expressed on endothelial cells and cells of the immune system. -

Cd11a/Integrin Alpha L Polyclonal Antibody
For Research Use Only CD11a/Integrin Alpha L Polyclonal antibody www.ptgcn.com Catalog Number:15574-1-AP 1 Publications Catalog Number: GenBank Accession Number: Recommended Dilutions: Basic Information 15574-1-AP BC008777 IHC 1:50-1:500 Size: GeneID (NCBI): 187 μg/ml 3683 Source: Full Name: Rabbit integrin, alpha L (antigen CD11A Isotype: (p180), lymphocyte function- IgG associated antigen 1; alpha polypeptide) Purification Method: Antigen affinity purification Calculated MW: 129 kDa Immunogen Catalog Number: AG7920 Applications Tested Applications: Positive Controls: IHC,ELISA IHC : human tonsillitis tissue; Cited Applications: WB Species Specificity: human Cited Species: human Note-IHC: suggested antigen retrieval with TE buffer pH 9.0; (*) Alternatively, antigen retrieval may be performed with citrate buffer pH 6.0 CD11a, also known as integrin αL or LFA-1α, is a 170-180 kDa transmembrane glycoprotein that forms a Background Information heterodimer with CD18 (PMID: 7027264; 1672643). CD11a/CD18 (LFA-1) is expressed by all leukocytes and mediates cell adhesion through interactions with its ligands, intercellular adhesion molecule 1 (ICAM-1), ICAM-2, and ICAM-3 (PMID: 7479767). CD11a/CD18 also functions in lymphocyte costimulatory signaling (PMID: 1972160). Notable Publications Author Pubmed ID Journal Application Ceshu Gao 34022890 J Neuroinflammation WB Storage: Storage Store at -20ºC. Stable for one year after shipment. Storage Buffer: PBS with 0.02% sodium azide and 50% glycerol pH 7.3. Aliquoting is unnecessary for -20ºC storage For technical support and original validation data for this product please contact: This product is exclusively available under Proteintech T: 4006900926 E: [email protected] W: ptgcn.com Group brand and is not available to purchase from any other manufacturer. -

Comparison of Acute Proton, Photon, and Low-Dose Priming Effects On
Tian et al. Fibrogenesis & Tissue Repair 2013, 6:4 http://www.fibrogenesis.com/content/6/1/4 RESEARCH Open Access Comparison of acute proton, photon, and low-dose priming effects on genes associated with extracellular matrix and adhesion molecules in the lungs Jian Tian1,3*†, Sisi Tian2 and Daila S Gridley1† Abstract Background: Crew members on space missions inevitably are exposed to low background radiation and can receive much higher doses during solar particle events (SPE) that consist primarily of protons. Ionizing radiation could cause lung pathologies. Cell adhesion molecules (CAM) are believed to participate in fibrogenesis. Interactions between CAM and extracellular matrix (ECM) affect epithelial repair mechanisms in the lung. However, there are very limited data on biological effects of protons on normal lung tissue. Numerous reports have shown that exposure to low-dose/low-dose-rate (LDR) radiation can result in radioadaptation that renders cells more resistant to subsequent acute radiation. The goal of this study was to compare expression of genes associated with ECM and CAM, as well as critical profibrotic mediators, in mouse lungs after acute irradiation with photons and protons, and also determine whether pre-exposure to LDR γ-rays induces an adaptive effect. Results: Overall, a marked difference was present in the proton vs. photon groups in gene expression. When compared to 0 Gy, more genes were affected by protons than by photons at both time points (11 vs. 6 on day 21 and 14 vs. 8 on day 56), and all genes affected by protons were upregulated. Many genes were modulated by LDR γ-rays when combined with photons or protons. -

Gene Expression Targets
Supplementary Table S1: Gene expression targets Cell Functional Probe ID Gene ID Gene Descriptions type Category Antigen-dependent activation MmugDNA.32422.1.S1_at CD180 CD180 molecule B 1 MmugDNA.18254.1.S1_at CD19 CD19 molecule B 1 MmugDNA.14306.1.S1_at CD1C CD1c molecule B 1 MmugDNA.20774.1.S1_at CD274 CD274 molecule B 1 MmugDNA.19231.1.S1_at CD28 CD28 molecule B 1 MmuSTS.3490.1.S1_at CD72 CD72 molecule B 1 MmugDNA.37827.1.S1_at CD79B CD79b molecule, immunoglobulin- associated beta B 1 MmugDNA.29916.1.S1_at CD80 CD80 molecule B 1 MmugDNA.6430.1.S1_at CD83 CD83 molecule B 1 MmugDNA.32699.1.S1_at CD86 CD86 molecule B 1 MmugDNA.19832.1.S1_at CIITA class II, major histocompatibility complex, transactivator B 1 MmuSTS.3942.1.S1_at CR2 complement component receptor 2 B 1 MmugDNA.25496.1.S1_at CTLA4 cytotoxic T-lymphocyte-associated protein 4 B 1 MmugDNA.5901.1.S1_at FAS Fas cell surface death receptor B 1 MmugDNA.8857.1.S1_at FASLG Fas ligand (TNF superfamily, member 6) B 1 MmugDNA.27012.1.S1_at FKBP2 FK506 binding protein 2, 13kDa P 1 MmugDNA.38565.1.S1_at FOS FBJ murine osteosarcoma viral oncogene homolog B 1 MmuSTS.165.1.S1_at ID3 inhibitor of DNA binding 3 B 1 MmugDNA.20492.1.S1_at IGBP1 immunoglobulin (CD79A) binding protein 1 B 1 MmugDNA.2488.1.S1_at IGHD immunoglobulin heavy constant delta B 1 MmuSTS.4350.1.S1_at IGHM immunoglobulin heavy constant mu B 1 MmunewRS.438.1.S1_at IL2 interleukin 2 B 1 MmugDNA.42882.1.S1_x_at SPN sialophorin B,P 1 MmuSTS.4526.1.S1_at SYK spleen tyrosine kinase B 1 MmuSTS.4672.1.S1_at TNF tumor necrosis factor