Inverse Association Between 1,5-Anhydroglucitol and Neonatal Diabetic Complications
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Effect of Continuous Glucose Monitoring on Hypoglycemia in Type 1 Diabetes
Clinical Care/Education/Nutrition/Psychosocial Research ORIGINALDiabetes ARTICLE Care Publish Ahead of Print, published online February 19, 2011 Effect of continuous glucose monitoring on hypoglycemia in type 1 diabetes 1 2 TADEJ BATTELINO, MD, PHD REVITAL NIMRI, MD especially useful in patients with hypogly- 2 3 MOSHE PHILLIP, MD PER OSKARSSON, MD, PHD 1 3 cemia unawareness and/or frequent epi- NATASA BRATINA, MD, PHD JAN BOLINDER, MD, PHD sodes of hypoglycemia (18). However, the hypoglycemia preventive effect of con- tinuous glucose monitoring has not been — OBJECTIVE To assess the impact of continuous glucose monitoring on hypoglycemia in established. Therefore, we designed a ran- people with type 1 diabetes. domized, controlled, multicenter clinical RESEARCH DESIGN AND METHODS—In this randomized, controlled, multicenter trial to evaluate the effect of continuous study, 120 children and adults on intensive therapy for type 1 diabetes and a screening level of glucose monitoring on hypoglycemia in glycated HbA1c ,7.5% were randomly assigned to a control group performing conventional children and adults with type 1 diabetes. home monitoring with a blood glucose meter and wearing a masked continuous glucose monitor every second week for five days or to a group with real-time continuous glucose monitoring. The primary outcome was the time spent in hypoglycemia (interstitial glucose concentration ,63 RESEARCH DESIGN AND mg/dL) over a period of 26 weeks. Analysis was by intention to treat for all randomized patients. METHODS RESULTS—The time per day spent in hypoglycemia was significantly shorter in the contin- uous monitoring group than in the control group (mean 6 SD 0.48 6 0.57 and 0.97 6 1.55 Patients Patients aged between 10 and 65 years h/day, respectively; ratio of means 0.49; 95% CI 0.26–0.76; P = 0.03). -

Blood Glucose Monitoring in Type 1 Diabetes (Adults)
FACTSHEET | JUNE 2021 Blood Glucose Monitoring in type 1 diabetes (adults) RSS Diabetes Service Blood glucose goes up and down during the day depending on carbohydrate intake, physical activity, insulin doses, wellbeing and stressors. Blood glucose results tend to be lower before meals and higher after meals. Blood glucose monitoring guides your self-care. The first step in blood glucose monitoring is knowing your blood glucose target ranges. What are the recommended blood glucose (BG) targets For most people with type 1 diabetes, it is recommended that blood glucose be as close to normal as possible to reduce the risk of long term complications. In general, recommended blood glucose targets are: Time Target BG Fasting and before meals 4.0 – 8.0mmol/L 2 hours after meals 4.0 – 10.0mmol/L However, for some people (e.g. infants and young children, the aged, those with impaired hypoglycaemia awareness or other health conditions), blood glucose targets will need to be set higher. If you are pregnant or trying to get pregnant, your blood glucose targets will be set lower. When should I test my blood glucose (BG)? Your blood glucose levels will indicate if your diabetes is well managed or if changes are needed. Blood glucose results overnight or before breakfast tell you how well your diabetes is controlled overnight by the basal insulin. Blood glucose results before lunch, before the evening meal and at bedtime tell you if your meal time rapid acting insulin doses are correct. Extra tests are recommended if you: • feel unwell, as part of your sick day action plan • are planning some physical activity, during and after physical activity • are using machinery • are about to drive • are concerned about unstable, unexpected or unexplainable results. -

GESTATIONAL DIABETES TESTING and TREAMENT Accepted: August 2015 Updated: August 2015
Boston Medical Center Maternity Care Guideline: GESTATIONAL DIABETES TESTING AND TREAMENT Accepted: August 2015 Updated: August 2015 INTRODUCTION: Definition: Gestational Diabetes (GDM) is impaired glucose tolerance with first onset or recognition during pregnancy. Prevalence: 4.6-9.2% of US pregnancies are affected by GDM. An increased prevalence of GDM is found among Hispanic, African American, Native American, Asian, and Pacific Islander women.1 Maternal Risk factors for GDM development include: o Personal history of GDM o Obesity (BMI≥30) o PCOS o Impaired glucose tolerance o Glycosuria early in pregnancy o Strong family history of diabetes (one first degree relative, or more than one second degree relative) o Previous macrosomic infant. o Previous unexplained third trimester loss or neonatal death. Maternal/fetal risks of GDM diagnosis: GDM is associated with significant maternal and neonatal morbidity. Maternal risks include development of hypertensive disorders and preeclampsia and development of type 2 diabetes mellitus later in life (50% develop DM within 20years, 60% of Latinas within 5years postpartum).1 Neonatal risks include large for gestational age, macrosomia, shoulder dystocia. , stillbirth, and newborn morbidity including hypoglycemia and respiratory distress. Obstetrical risks include risks associated with macrosomia such as increased rate of operative vaginal birth and cesarean section, brachial plexus injury, fracture, and neonatal depression. DIAGNOSIS: All pregnant women will be screened for gestational diabetes, with the exception of women who already carry a diagnosis of type 1 or type 2 diabetes. The diagnosis will be based on specific criteria. Early Screening for High Risk Populations: Pregnant women who meet the following criteria should be screened as early as possible, preferably at the first prenatal visit. -

Blood Glucose Monitoring
Blood Glucose Monitoring Many people are frightened to check their blood sugar levels because they do not want to see levels that are higher or lower than their target range. However, checking your blood sugars puts you in control of your diabetes. Remember, your blood sugar levels are what they are whether you know about them or not but when you know what they are; you can make adjustments, if needed. Monitoring your blood sugar levels is the most accurate way of you seeing the effectiveness of your lifestyle changes and medications. Monitoring provides you with the ability to identify possible causes of blood sugar fluctuations. Tips for Monitoring • Wash your hands with soap in warm water before checking your blood sugar • Ensure your test strips have not expired • Remember to calibrate your meter (if your meter requires this) • Use the sides of your fingers and remember to use different fingers o Do not use alternate site testing if you think your blood sugar is very high or low – finger checks give you the most accurate result • Discuss the with your healthcare team the best times to check your blood sugar level – which may include: o Prior to your meal and / or 2 hours after the meal o Overnight o If you feel unwell • Record your results in a logbook; this will help you to identify patterns in your levels and make the required changes to get your blood sugar back to your target range; bring your blood sugar records and meter(s) to your healthcare visit - it will help you and your healthcare team to determine your diabetes management needs. -
Hyperglycemia – Prediabetes
North Dakota Family Planning Program Clinical Protocol Manual Health Maintenance – HM 8 Hyperglycemia – Prediabetes DEFINITION Hyperglycemia is an excess of glucose in the blood. Prediabetes is a condition when blood glucose levels are higher than normal but not yet high enough to be diagnosed as diabetes. (Prediabetes is also known as Impaired Glucose Tolerance (IGT) or Impaired Fasting Glucose (IFG) depending on which test was used to detect it. IGT uses the oral glucose tolerance test and IFG uses the fasting plasma glucose test.) SUBJECTIVE May include: 1. No symptoms 2. Family history of diabetes 3. Weight changes 4. Fatigue, blurred vision, recurrent vulvovaginal candidiasis 5. History of gestational diabetes or birth weight of newborn 9 lbs. or greater 6. Polydipsia, polyphagia, or polyuria 7. 20% over ideal body weight; sedentary lifestyle 8. Decreased wound healing 9. Race (higher incidence of diabetes among American Indian, Hispanic, and African American population) 10. History of hypertension 11. History of decreased HDL and increased triglycerides 12. History of Polycystic Ovarian Syndrome or Metabolic Syndrome 13. History of antipsychotic medication therapy OBJECTIVE May include: 1. Blood pressure 2. Height and weight 3. Complete physical exam. Acanthosis Nigricans may be noted in axillae, groin, and/or neck LABORATORY May refer for: 1. Hyperglycemia a. lab of choice may include one or more of the following: i. Fasting plasma glucose (FPG) a) Prediabetes: FPG between 100 mg/dl to 125 mg/dl b) Diabetes: FPG is > 126 mg/dl ii. Oral Glucose Tolerance Test (OGTT) a) Prediabetes: OGTT 140mg/dl to 199 mg/dl b) Diabetes: OGTT 200mg/dl or higher iii. -

1,5 Anhydroglucitol in Gestational Diabetes Mellitus
Journal of Diabetes and Its Complications 33 (2019) 231–235 Contents lists available at ScienceDirect Journal of Diabetes and Its Complications journal homepage: WWW.JDCJOURNAL.COM 1,5 Anhydroglucitol in gestational diabetes mellitus Thyparambil Aravindakshan Pramodkumar a, Ramamoorthy Jayashri a, Kuppan Gokulakrishnan a, Kaliyaperumal Velmurugan a, Rajendra Pradeepa a, Ulagamathesan Venkatesan a, Ponnusamy Saravanan b, Ram Uma c, Ranjit Mohan Anjana a, Viswanathan Mohan a,⁎ a Madras Diabetes Research Foundation & Dr. Mohan's Diabetes Specialities Centre, WHO Collaborating Centre for Non-communicable Diseases Prevention and Control, ICMR Centre for Advanced Research on Diabetes, Gopalapuram, Chennai, India b Division of Health Sciences, Warwick Medical School, University of Warwick, Coventry, United Kingdom c Department of Obstetrics and Gynecology, Seethapathy Clinic and Hospital, Chennai, Tamil Nadu, India article info abstract Article history: Objective: 1,5 Anhydroglucitol (1,5 AG) is reported to be a more sensitive marker of glucose variability and short- Received 25 April 2018 term glycemic control (1–2 weeks) in patients with type1 and type 2 diabetes. However, the role of 1,5 AG in ges- Received in revised form 17 October 2018 tational diabetes mellitus (GDM) is not clear. We estimated the serum levels of 1,5 AG in pregnant women with Accepted 28 November 2018 and without GDM. Available online 5 December 2018 Methods: We recruited 220 pregnant women, 145 without and 75 with GDM visiting antenatal clinics in Tamil Nadu in South India. Oral glucose tolerance tests (OGTTs) were carried out using 82.5 g oral glucose (equivalent Keywords: to 75 g of anhydrous glucose) and GDM was diagnosed based on the International Association of Diabetes and 1,5 Anhydroglucitol Gestational diabetes mellitus Pregnancy Study Group criteria. -

Download PDF File
Ginekologia Polska 2018, vol. 89, no. 1, 25–29 Copyright © 2018 Via Medica ORIGINAL PAPER / OBSTETRICS ISSN 0017–0011 DOI: 10.5603/GP.a2018.0005 Does reactive hypoglycemia during the 100 g oral glucose tolerance test adversely affect perinatal outcomes? Ilhan Bahri Delibas1, Sema Tanriverdi2, Bulent Cakmak1 1Department of Obstetrics and Gynecology, Gaziosmanpasa University, Tokat, Turkey 2Neonatalogy Clinic, Merkez Efendi State Hospital, Manisa, Turkey ABSTRACT Objectives: To determine whether pregnant women who have reactive hypoglycemia during the 100 g oral glucose toler- ance test (OGTT) are at an increased risk of poor pregnancy outcomes. Material and methods: We retrospectively analyzed perinatal data from 413 women who underwent a 3 h OGTT at 24–28 weeks of gestation and gave birth in our clinics between January 2012 and December 2014. Results: According to OGTT results, the majority of the subjects were normoglycemic (n = 316, 76.5%), while 49 (11.9%) were diagnosed with gestational diabetes, and 33 (8.0%) had single high glucose values. Reactive hypoglycemia was de- tected in only 15 patients (3.6%). The mean age of the women in the reactive hypoglycemia group was significantly lower than that of the women in the gestational diabetes and single high glucose value groups (26.4 ± 4.4 years, 31.4 ± 5.4 years, and 31.8 ± 4.3 years, respectively; p < 0.05). The newborns of the women in the reactive hypoglycemia group had higher rates of APGAR scores < 7, increased admission to the neonatal intensive care unit (NICU), and lower birth weights compared with the other groups (p < 0.001, p < 0.001, and p = 0.009, respectively). -

Diabetes in Pediatrics: Advances in Treatment and Technologies
7/5/2019 Diabetes in Pediatrics: Advances in Treatment and Technologies Lisa Swartz Topor, MD, MMSc Pediatric Endocrinology July 9, 2019 Objectives • Evaluate children presenting with new onset diabetes mellitus • Describe recent advances in insulin pump therapy • Explore how continuous glucose monitors are used in diabetes management Types of Diabetes Mellitus • Type 1A Immune-mediated • Type 1B Insulin-deficient, not autoimmune • Type 2 Combined insulin resistance and insulin secretory deficiency • Secondary Cystic fibrosis; pancreatitis; endocrinopathies (e.g. Cushing syndrome, acromegaly); drug-induced (e.g. glucocorticoids) • Gestational First recognized/onset during pregnancy • Monogenic Maturity-onset diabetes of the young (MODY); insulin signaling defects; insulin gene mutations 1 7/5/2019 Criteria for Diagnosis of Diabetes Mellitus (Non-Pregnant Individuals) Plasma glucose 200 mg/dl at any time plus classic symptoms such as increased urination (polyuria), increased thirst (polydipsia), and weight loss or Fasting plasma glucose 126 mg/dl (fasting 8 hours after last meal) or 2-hour plasma glucose 200 mg/dl during an oral glucose tolerance test (75 g) or Hemoglobin A1c > 6.5% Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 2010;33(Supp 1):S62-S69. Type 1 Diabetes in Youth Diagnosis of Type 1 Diabetes • Clinical features • Polydipsia, polyuria, polyphagia, weight loss • Absence of signs of insulin resistance (acanthosis nigricans)* • Age, ethnicity, family history • Glucose/A1C criteria • Presence of antibodies -
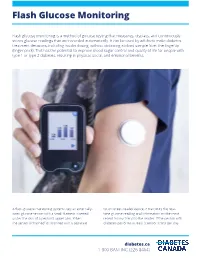
Flash Glucose Monitoring
Flash Glucose Monitoring Flash glucose monitoring is a method of glucose testing that measures, displays, and continuously stores glucose readings that are recorded automatically. It can be used by adults to make diabetes treatment decisions, including insulin dosing, without obtaining a blood sample from the fingertip (finger prick). This has the potential to improve blood sugar control and quality of life for people with type 1 or type 2 diabetes, resulting in physical, social, and emotional benefits. A flash glucose monitoring system uses an externally- touchscreen reader device, it transmits the real- worn glucose sensor with a small filament inserted time glucose reading and information on the most under the skin of a person’s upper arm. When recent 8-hour trend to the reader. If the person with the sensor is “flashed” or scanned with a separate diabetes performs at least 3 sensor scans per day diabetes.ca 1-800-BANTING (226-8464) at approximately 8-hour intervals, the flash glucose monitor can record 24-hour glucose profiles. A sensor can be worn continuously for up to 14 days. Blood glucose monitoring gives people living with diabetes a more complete picture of their glucose control, which can lead to better short and long-term treatment decisions and health outcomes. It can help them identify when their blood sugar is trending down, which allows for appropriate and timely action to be taken to avoid hypoglycemia (low blood sugar). It can also provide early indication of hyperglycemia (high blood sugar) over the course of the day and prompt adjustments to medications, activity, and food intake to help achieve blood sugar targets. -

Diabetic Testing
PRE -ASSESSMENT Diabetic Testing Before CCOHTA decides to undertake a health technology assessment, a pre-assessment of the literature is performed. Pre-assessments are based on a limited literature search; they are not CCOHTA extensive, systematic reviews of the literature. They are provided here as a quick guide to important, No. 34 current assessment information on this topic. Readers are cautioned that the pre-assessments have May 2004 not been externally peer reviewed. Introduction Diabetes is prevalent in 5% of the Canadian population.1 Type 2 non-insulin dependent diabetes mellitus (NIDDM) accounts for 85% to 90% of patients with diabetes mellitus. Patients with diabetes have an increased risk for developing complications from cardiovascular (i.e., heart, cerebrovascular and peripheral vascular) disease including neuropathy (leading to limb amputation), retinopathy (leading to blindness) and nephropathy (leading to dialysis and transplant). The clinical management of diabetes ranges from surveillance and primary prevention (preventing disease occurrence) to secondary prevention and management of complications. Regular treatment and the measurement of blood lipid parameters, blood pressure and glycemia used to modify treatment plans are encouraged in patients with type 1 (insulin-dependent) or type 2 diabetes (Figure 1).1 Figure 1: Diagnosis and care of diabetes based on glycemic control Management of Diabetes Treating:Treating 1. What is the evidence that LifestyleEducation –about physical improved glycemic control nutritional,lifestyle, nutrition and and delays or leads to fewer disease knowledge complications education Diagnosis and and screening screening AntihyperglycemicAntihyperglycemic medications Glycemic Complications Type 1 control Type 1 or death or typetype 2 2 diabetes diabetes Testing:Testing GlucoseFasting in bloodplasma or plasmaglucose Fructosamine 2. -

Metabolic Parameters in Patients with Suspected Reactive Hypoglycemia
Journal of Personalized Medicine Article Metabolic Parameters in Patients with Suspected Reactive Hypoglycemia Marianna Hall 1,2,* , Magdalena Walicka 2,3, Mariusz Panczyk 4 and Iwona Traczyk 1 1 Department of Human Nutrition, Faculty of Health Sciences, Medical University of Warsaw, 01-445 Warsaw, Poland; [email protected] 2 Department of Internal Diseases, Endocrinology and Diabetology, Central Clinical Hospital of the Ministry of Internal Affairs and Administration in Warsaw, 02-507 Warsaw, Poland; [email protected] 3 Department of Human Epigenetics, Mossakowski Medical Research Institute Polish Academy of Sciences, 02-106 Warsaw, Poland 4 Department of Education and Research in Health Sciences, Faculty of Health Sciences, Medical University of Warsaw, 02-091 Warsaw, Poland; [email protected] * Correspondence: [email protected] Abstract: Background: It remains unclear whether reactive hypoglycemia (RH) is a disorder caused by improper insulin secretion, result of eating habits that are not nutritionally balanced or whether it is a psychosomatic disorder. The aim of this study was to investigate metabolic parameters in patients admitted to the hospital with suspected RH. Methods: The study group (SG) included non-diabetic individuals with symptoms consistent with RH. The control group (CG) included individuals without hypoglycemic symptoms and any documented medical history of metabolic disorders. In both groups the following investigations were performed: fasting glucose and insulin levels, Homeostatic Model Assessment for Insulin Resistance (HOMA-IR), 75 g five-hour Oral Glucose Tolerance Test (OGTT) with an assessment of glucose and insulin and lipid profile evaluation. Additionally, Mixed Meal Tolerance Test (MMTT) was performed in SG. -

Clinical Usefulness of the Measurement of Serum Fructosamine in Childhood Diabetes Mellitus
Original article http://dx.doi.org/10.6065/apem.2015.20.1.21 Ann Pediatr Endocrinol Metab 2015;20:21-26 Clinical usefulness of the measurement of serum fructosamine in childhood diabetes mellitus Dong Soo Kang, MD1, Purpose: Glycosylated hemoglobin (HbA1c) is often used as an indicator of glucose Jiyun Park, MD1, control. It usually reflects the average glucose levels over two to three months, and Jae Kyung Kim, PhD2, is correlated with the development of long-term diabetic complications. However, Jeesuk Yu, MD, PhD1 it can vary in cases of hemoglobinopathy or an altered red blood cell lifespan. The serum fructosamine levels reflect the mean glucose levels over two to three weeks. 1 This study was designed to determine the clinical usefulness of the combined Departments of Pediatrics and 2Laboratory Medicine, Dankook measurement of serum fructosamine and HbA1c in the management of childhood University Hospital, Dankook diabetes mellitus and the correlation between them. University College of Medicine, Methods: Clinical data on 74 Korean children and adolescents with diabetes Cheonan, Korea mellitus who were under management at the Department of Pediatrics of Dankook University Hospital were evaluated. Their fructosamine and HbA1c levels were reviewed based on clinical information, and analyzed using IBM SPSS Statistics ver. 21. Results: Their HbA1c levels showed a strong correlation with their fructosamine levels (r=0.868, P<0.001). The fructosamine level was useful for the prompt evaluation of the recent therapeutic efficacy after the change in therapeutic modality. It was also profitable in determining the initial therapeutics and for the estimation of the onset of the disease, such as fulminant diabetes.