Prevalence of Chronic Kidney Disease and Its Association With
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Statistical Diary, Uttar Pradesh-2020 (English)
ST A TISTICAL DIAR STATISTICAL DIARY UTTAR PRADESH 2020 Y UTT AR PR ADESH 2020 Economic & Statistics Division Economic & Statistics Division State Planning Institute State Planning Institute Planning Department, Uttar Pradesh Planning Department, Uttar Pradesh website-http://updes.up.nic.in website-http://updes.up.nic.in STATISTICAL DIARY UTTAR PRADESH 2020 ECONOMICS AND STATISTICS DIVISION STATE PLANNING INSTITUTE PLANNING DEPARTMENT, UTTAR PRADESH http://updes.up.nic.in OFFICERS & STAFF ASSOCIATED WITH THE PUBLICATION 1. SHRI VIVEK Director Guidance and Supervision 1. SHRI VIKRAMADITYA PANDEY Jt. Director 2. DR(SMT) DIVYA SARIN MEHROTRA Jt. Director 3. SHRI JITENDRA YADAV Dy. Director 3. SMT POONAM Eco. & Stat. Officer 4. SHRI RAJBALI Addl. Stat. Officer (In-charge) Manuscript work 1. Dr. MANJU DIKSHIT Addl. Stat. Officer Scrutiny work 1. SHRI KAUSHLESH KR SHUKLA Addl. Stat. Officer Collection of Data from Local Departments 1. SMT REETA SHRIVASTAVA Addl. Stat. Officer 2. SHRI AWADESH BHARTI Addl. Stat. Officer 3. SHRI SATYENDRA PRASAD TIWARI Addl. Stat. Officer 4. SMT GEETANJALI Addl. Stat. Officer 5. SHRI KAUSHLESH KR SHUKLA Addl. Stat. Officer 6. SMT KIRAN KUMARI Addl. Stat. Officer 7. MS GAYTRI BALA GAUTAM Addl. Stat. Officer 8. SMT KIRAN GUPTA P. V. Operator Graph/Chart, Map & Cover Page Work 1. SHRI SHIV SHANKAR YADAV Chief Artist 2. SHRI RAJENDRA PRASAD MISHRA Senior Artist 3. SHRI SANJAY KUMAR Senior Artist Typing & Other Work 1. SMT NEELIMA TRIPATHI Junior Assistant 2. SMT MALTI Fourth Class CONTENTS S.No. Items Page 1. List of Chapters i 2. List of Tables ii-ix 3. Conversion Factors x 4. Map, Graph/Charts xi-xxiii 5. -

1951 Rae Bareli ,District
I Census of India, 1951 DISTRICT CENSUS HANDBOOK UTTAR PRADESH 42-RAE BARELl DISTRICT l ALLAliABAD: SUPERINTENDENT, PRINTING AND ST~TIONERY> U'ITAR PRADESH, -INDlA 1960 __ -----------------------------------.------------1 '- .... _----- -- -- DISTRICT CENSUS HANDBOOK 1951 RAE BARELI ,DISTRICT FOREWORD Several Stat~~, including Uttar Pradesh, have been publishing village statistics by districts at each census. In 1941 they were, published in U. P. ul!der the title "District Census Statistics" with a separate volume for each district. In the 1951 census, when the tabulation has been more elaborate than ever in view of the require" ments of the country, the districtlWise volume has been expanded into a "District Census Handbook", which now contains the District Census Tables (furnishing data wi~ break .. up for census tract~ within the district), the District 'Index of Non" ,:~.< •. agried1mral Occupations, agricultural statistics from 1901102 to 1950/51 and other mi~cellaneous statistics in addition to the usual village population statistics. The village popuiation statistics also are given in an elaborate form giving the division of the population among eight livelihood classes and other details. 2. It may be added here that a separate set of districtlWise volumes giving only population figures of rural areas by villages and of urban areas by wards and mohallas and entitled "District Population Statistics" has already been published. This separate series was necessitated by the urgent requirements of the U. P. Government for elections to local bodies. 3. The number of Distr1ct Census Handbooks printed so far is fo[ty'oine Special arrangements for speeding up the printing have now been made and it is hoped that the remaining Handbooks wilt be printed before the end of 1955. -

Lucknow Zone CSC List.Xlsx
Lucknow Zone CSC List Sl. Grampanchayat District Block Name Village/CSC name Pincode Location VLE Name Contact No No. Village Name 1 Sultanpur Sultanpur4 JAISINGHPUR(R) 228125 ISHAQPUR DINESH ISHAQPUR 730906408 2 Sultanpur Baldirai Bhawanighar 227815 Bhawanighar Sarvesh Kumar Yadav 896097886 3 Hardoi HARDOI1 Madhoganj 241301 Madhoganj Bilgram Road Devendra Singh Jujuvamau 912559307 4 Balrampur Balrampur BALRAMPUR(U) 271201 DEVI DAYAL TIRAHA HIMANSHU MISHRA TERHI BAZAR 912594555 5 Sitapur Sitapur Hargaon 261121 Hargaon ashok kumar singh Mumtazpur 919283496 6 Ambedkar Nagar Bhiti Naghara 224141 Naghara Gunjan Pandey Balal Paikauli 979214477 7 Gonda Nawabganj Nawabganj gird 271303 Nawabganj gird Mahmood ahmad 983850691 8 Shravasti Shravasti Jamunaha 271803 MaharooMurtiha Nafees Ahmad MaharooMurtiha 991941625 9 Badaun Budaun2 Kisrua 243601 Village KISRUA Shailendra Singh 5835005612 10 Badaun Gunnor Babrala 243751 Babrala Ajit Singh Yadav Babrala 5836237097 11 Bareilly Bareilly2 Bareilly Npp(U) 243201 TALPURA BAHERI JASVEER GIR Talpura 7037003700 12 Bareilly Bareilly3 Kyara(R) 243001 Kareilly BRIJESH KUMAR Kareilly 7037081113 13 Bareilly Bareilly5 Bareilly Nn 243003 CHIPI TOLA MAHFUZ AHMAD Chipi tola 7037260356 14 Bareilly Bareilly1 Bareilly Nn(U) 243006 DURGA NAGAR VINAY KUMAR GUPTA Nawada jogiyan 7037769541 15 Badaun Budaun1 shahavajpur 243638 shahavajpur Jay Kishan shahavajpur 7037970292 16 Faizabad Faizabad5 Askaranpur 224204 Askaranpur Kanchan ASKARANPUR 7052115061 17 Faizabad Faizabad2 Mosodha(R) 224201 Madhavpur Deepchand Gupta Madhavpur -

Raibareilly Dealers Of
Dealers of Raibareilly Sl.No TIN NO. UPTTNO FIRM - NAME FIRM-ADDRESS 1 09152400002 RB0012368 RAIBARALI GAN HOUSE KASARGANJ RAIBARALI 2 09152400016 RB0022220 S.VLAYAT ALI AND SONS KAISER GANJ,RAEBARELI 3 09152400021 RB0033437 SARARE PLASTIC PRV.LTD. SULTAN PUR ROAD,RAEBARELI 4 09152400040 RB0054810 SAHU MEDICAL STORE JAYAS RAIBARELI 5 09152400049 RB0035506 GARG INDUSTRISE SULTANPUR ROAD RAIBARELI 6 09152400054 RB0035025 BOMBAY TIMBER WORKS MANDI MANDI SAMITI ROAD RAIBARELI SAMITI 7 09152400068 RB0036205 GUPTA RADIOS HOSPITAL CHAURAHA RAEBARELI 8 09152400087 RB0013107 SHUKLA IORN TREDERS BACHHRAWAN,RAEBARELI 9 09152400092 RB0033312 TRIPATHI BROS. MEDI8CAL STORES HOSPITAL CHAURAHA,RAEBARELI 10 09152400101 RB0030556 RAIBARALI CYCLE INDUSTRIES SUPER MARKET RAIBARALI 11 09152400115 RB0038930 AGRAWAL AGENCY JAYAS RAIBARELI 12 09152400134 RB0040998 KRISHNA CHANDRA AGARWAL JAYAS RAEBARELI 13 09152400148 RB0044238 BOMBAY CYCLE MART KACHARI ROAD RBL 14 09152400153 RB0030657 JAIN MEDICAL STORE JAYAS RAIBARELI 15 09152400167 RB0046368 SUDHIR BHARGAWA PRABHU TOWN RAIBARELI 16 09152400172 RB0047787 RAJPOOT CONTRACTION COMP. SATYA NAGAR RAIBARELI 17 09152400186 RB0046607 VIVED STORES JAYAS RBL 18 09152400191 RB0046393 SHREE DHAR ENTERPRISES SULTANPUR ROAD RAIBARELI 19 09152400200 RB0048348 KAUSAL BRICK FIELD RAJA FATEHPUR RAEBARELI 20 09152400209 RB0048401 FANCY GENERAL STORES JAIS RAIBARELI 21 09152400214 RB0048599 RABI MOTER STORE CIVIL LINES RAIBARELI 22 09152400228 RB0049629 VIJAY MACH. STORES TILOI RBL 23 09152400233 RB0050009 KULVIR SINGH BACHARAWA -

VISAKA INDUSTRIES LIMITED, RAE BARELI-2018 Minutes of Meeting of Public Hearing-28.06.2018
VISAKA INDUSTRIES LIMITED, RAE BARELI-2018 Minutes of Meeting of Public Hearing-28.06.2018 The proceedings (minutes) of the Public Hearing conducted at the premises of M/s Visaka Industries limited, Village: Kannawan, Post: Bachhrawan, Tehsil: Maharajganj, Dist.: Rae Bareli, at 10 AM on 28-06-2018 for the expansion of plant production capacity from the existing 1,20,000 TPA to 3,20,000 TPA (in Phase 1 – 40,000 TPA & in Phase 2 – 1,60,000 TPA) chaired under ADM – E (Administration), Rae Bareli. It has been proposed to increase the production capacity of the Asbestos Cement Sheet plant of M/s Visaka Industries limited located at Village: Kannawan, Post: Bachhrawan, Tehsil: Maharajganj, District: Rae Bareli from the existing 1,20,000 TPA to 3,20,000 TPA (in Phase 1 – 40,000 TPA & in Phase 2 – 1,60,000 TPA). The Member Secretary, U.P. Pollution Control Board, through the letter No. H02286/C-5/NOC/17, dated 16/05/2017, had requested the District Magistrate, Rae Bareli to schedule the dates for the Public Hearing between 20/06/2017 and 25/06/22017, to fix or approve a suitable venue & also requested the DM, Rae Bareli to chair the Public Hearing Panel or alternatively nominate a representative, not below the rank of Additional District Magistrate, Rae Bareli. For conducting the Public Hearing. In that regard, the Chief Environmental Officer (Circle 5), U.P. Pollution Control Board, Lucknow, through the letter No. H17958/C-5/NOC-23/Public Hearing/18, dated 04/04/2018, made such a request to fix the time, date & venue for conducting the Public Hearing. -
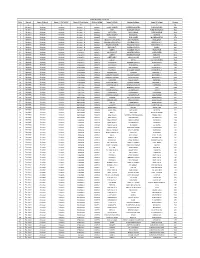
ASHA Data Base Raebareli Name of Population S.No
ASHA Data Base Raebareli Name Of Population S.No. District Name Of Block Name Of CHC/BPHC Name Of Sub-Centre ID No.of ASHA Name Of ASHA Husband's Name Name Of Village Covered 1 2 3 4 5 6 7 8 9 10 1 Raebareli Amawan Amawan Amawan - A 6101001 ACHAL KUMARI SURESH CHANDRA PASIN KODARA 1444 2 Raebareli Amawan Amawan Amawan - A 6101002 ANITA SANTOSH KUMAR PURE THAKURAIN 1500 3 Raebareli Amawan Amawan Amawan - A 6101003 ANITA DEVI SHIV KISHOR PURE SARDAR 1494 4 Raebareli Amawan Amawan Amawan - A 6101004 ANITA MAURYA RAM DAYAL AMAWAN 800 5 Raebareli Amawan Amawan Amawan - A 6101005 ARCHANA RAM SUMER MAKHDOOMPUR 1170 6 Raebareli Amawan Amawan Amawan - A 6101006 ARCHANA CHAUDARI SHIV PRASHAD RASULPUR 984 7 Raebareli Amawan Amawan Amawan - B 6101007 ARCHANA SRIVASTAVA LAVLESH YADAV HARIYAWAN - I 1086 8 Raebareli Amawan Amawan Amawan - B 6101008 ARCHANA TIWARI RAM DAS YADAV HARIYAWAN - II 1400 9 Raebareli Amawan Amawan Amawan - B 6101009 BHANUMATI DINESH MAURYA PADRAI 900 10 Raebareli Amawan Amawan Amawan - B 6101010 DEEPA KAMLESH YADAV RUKUNPUR 1500 11 Raebareli Amawan Amawan Ghuradeeh 6101011 DHARMAWATI CHANDRA BARAN GHURADEEH 1785 12 Raebareli Amawan Amawan Ghuradeeh 6101012 GEETA RAJENDRA SINGH DEDAIYA 1475 13 Raebareli Amawan Amawan Ghuradeeh 6101013 GEETA DEVI GNAGA SAGAR DUSAUTI 1700 14 Raebareli Amawan Amawan Ghuradeeh 6101014 GOLDI IRFAN MO PUR CHURAI 1125 15 Raebareli Amawan Amawan Ghuradeeh 6101015 JAGESWARI SURESH MAURYA SAMRAHADA 1300 16 Raebareli Amawan Amawan Ghuradeeh 6101016 JANAKSUTA RAM KEWAL OLIPUR 1340 17 Raebareli Amawan Amawan -

RAEBARELI PARLIAMENTARY CONSTITUENCY Uttar Pradesh, the Most Populous State of Nation Is Served by North Central Railway Along with Northern, North Eastern M
NORTH CENTRAL RAILWAY RAEBARELI PARLIAMENTARY CONSTITUENCY Uttar Pradesh, the most populous state of Nation is served by North Central Railway along with Northern, North Eastern M. C. Chauhan and East Central Railways. Indian Railways (IR) plays a very General Manager important role in development of the state and provides North Central Railway connectivity for freight and passengers, including international tourists. IR has a history of more than 160 years in the state and has the highest route kilometers - 9100 route kilometers - within it, which is 14% of entire railway network. IR has made Uttar Pradesh proud by giving it Nation's first semi high speed train Gatimaan Express, which initially ran between Agra and Delhi. It has now been extended upto Jhansi with effect from 01.04.2018 - a move that will boost tourism in bundelkhand region of Uttar Pradesh. Efforts for further development of railway infrastructure in Uttar Pradesh have been intensified in last four years with 376% more investment in comparison to previous years. Results of such a massive investment are becoming visible with enhanced infrastructure and modern passenger amenities. Soon the era of congestion and inconvenience will be a matter of past and a new Railway equipped with state-of-the-art facilities will be able to serve the citizens with greater level of satisfaction. The longest stretch of the under construction Eastern Dedicated Freight Corridor i.e. almost 57% of the entire length lies in Uttar Pradesh from Pt. Deen Dayal Upadhyaya Jn. to Ghaziabad. In this brochure, we have compiled the important contributions and achievements of Indian Railways in the development of the RAEBARELI constituency of Uttar Pradesh in the last four years. -

Brief Industrial Profile of District Raebareli
Government of India Ministry of MSME Brief Industrial Profile of District Raebareli MSME-DEVELOPMENT INSTITUTE Ministry of MSME, Govt. of India 107, Industrial Estate, Kalpi Road, Kanpur – 208012. (U.P.) Phone: 0512-2295070-73 Tele/Fax: 0512-2240143 Email: [email protected] Website: msmedikanpur.gov.in CONTENTS S.No. Topic Page No. 1. General Characteristics of the District 1 1.1 Location & Geographical Area 1 1.2 Topography 2 1.3 Availability of Minerals. 2 1.4 Forest 2 1.5 Administrative set up 3 2. District at a glance 4-6 2.1 Existing Status of Industrial Area in the Raebareli 7 3. Industrial Scenario of Raebareli 8 3.1 Industry at a Glance 8 3.2 Year Wise Trend of Units Registered 9 3.3 Details of Existing Micro & Small Enterprises & Artisan Units in the 10 District 3.4 Large Scale Industries / Public Sector Undertakings 11 3.5 Major Exportable Item 11 3.6 Growth Trend 11 3.7 Vendorisation / Ancillarisation of the Industry 11 3.8 Medium Scale Enterprises 11 3.8.1 List of the units in District Raebareli & near by area 12 3.8.2 Major Exportable Items 12 3.9 Service Enterprises 12-14 3.9.1 Potential Areas for Service Industry 14 3.10 Potential for new MSMEs 15 4. Existing Clusters of Micro & Small Enterprise 16 4.1 Detail of Major Clusters 16 4.1.1 Manufacturing Sector 16 4.1.2 Service Sector 16 4.2 Details of Identified Cluster 17-21 5. General issues raised by Industry Associations during the course of 22 meeting 6 Prospects of training Programmes during 2012-13 23 7. -

District Census Handbook, Rarbareli, Part XII-A, Series-25, Uttar Pradesh
CENSUS 1991 . ~'8jC11-25 SERIES-25 \3Cd\( ~ UTTAR PRADESH 'BPI-XII3T FART-XIlA "!JI~ q ~~Ix VILLAGE & TOWl'J Ri~~ICf)1 DIRECrrOR1[ F\Ji (Yf! \J1 ~ ~ 10 I 'i I Q f(19)ffii Cf) I DISTFJ:CT CENSUS :HANDBOOK FGi&11 '<1'Q~~(Yj1 DISTRICT RAEBARELI DIRECTOR OF CE:\SVS OPERATIONS UTTAR PRADESH 1. !H"C'11 q<rj I v 2. ~~ 3. ~ cpr JiI""l~?I 4. ~ m JOjt:?(CI~f ~ IX 5. ftrc;rr ,iF-PIcHI t:?ffi9Jf'«1CbI em ~ ~Rl61{i XIX 6. fq~c'l~uIIC'liCb recqu~ 1 7. ~- I(i) JiI'i~?I 3fh- lJl1'l fY1~~ICbI 1. '{iljC;:I~Cf) FclCbI{i ~-iSl'(Hlqi 16 26 2. XilljC;:I~Cf) FclcnlXi ~-l)lq~1 if) 34 3. ftljC:IRlct5 ~CI'>lft ~-Ji61"!I'JPi\J1 44 4. ftljt;IRlCf) FclCf)IXi ~-ffi59J"! 54 5. ftljC:IR1cp fClCl'>lfl ~-~ 64 6. ftljC:IRlCb fqcnlft ~-iSl615'<9J"! 70 7 . ftl j c;: Ifil Cb Fcl qJ! tl ~ g '<'tI ""G 9)'< 80 8. ftljC:IRlcp Fc1C1'>ltl ~-3P1icrr 92 9. tlljC:I RlCb FclCbltl ~-'tl"fficr 102 10. fllljC:lfilCb Rl4?ltl ~-XTi5r 116 11. tlljC:IRlCb FclCbltl ~-~ 128 12. tll~C:lfilcp RlCbltl ~-~ 148 13. ftllj41RlCb Fcl4?ltl ~-crtlcrt~r\ij 160 14. fil~C;:IRlCb FclCbltl ~-;SC'1&ji3'i 176 15. flllj4lfilCf) fClCblfl ~-\J1"IC19J\( 16. filjC:IRl¢ FclCblfi ~-tTg 192 17. flljC;:IRlCb fq4?lfi ~-~ 202 210 18 ftllj41~Cb fcl4?1tl ~- x=rcrtFr 19 flljG:lRlqj FclCf)ltl ~-;;hi1lgl'< 226 20 q;:r ml'f ~- I(ii) lJ11TI' ~ CjolfjwJi ~ 1. -

District Census Handbook, 39-Raebareli, Uttar Pradesh
CENSUS 1961 DISTRICT CENSUS HANDBOOK UTTAR PRADESH 39-RAEBARELI DISTRICT LUC~NOW: Superintendent. Printing and Stationery. (1. P. (India) 1965 CENSUS 1961 DISTRICT CENSUS HANDBOOK UTTAR PRADESH 39-RAEBARELI DISTRICT LUC~NOW: Superintendent. Printing and Stationery. (1. P. (India) 1965 81" IS' E N DISTRICT RAE BAREL' B I o \ :::> a. Z IS 15' ," DISTRICT HEADQUARTERS TAHSIL HEADOUAPJ'ERS B~OCK HEADOUARTERS 'DISTRICT BOUNDARV TAHSIL BOUNDARY RAILWAY LINE WITH STATION e.G. STATE HIGHWAY - LOCAL ROllO METALLED RIVER CANAL REST HOUSE ® POLICE STATION ® POST & TELEGRAPH OFFICE PT POST O~ICE PO HOSPITAL ~ DISPENSAHV ED MUNICIPAL TOWN Ie] TOWN ® ~=~=IL=L=A=G=E=A=BOIV~E~~=O=O=O=P=O=P=U=L=AT=I=O=N===O==~ __~~ ________________________~~ ____________________~J_~ __________ .J'N 45' lSI 81 JOI PREFACE District-wise village statistics have been published at most of thc_ Censuses. A list showing the population of villages in each district was published after the 1891 Census. No such list was brought out in 1901. In 1911 Village Directories were prepared for all districts, but could ~c published only for 13 on account of the out break of the First World War. At the 1921 Census they were published for all districts in the form of District Census Statistics. In 1931 they were compiled for all districts, but were not published owing to financial stringency, leading to loss of valuable data. A t the 1941 Census even though restricted tabulation was undertaken on account of the Second World War, yet the utility of District Census Statis tics was recognized and they were published. -
Sl No District Branch Name Bank Name
SL NO DISTRICT BRANCH NAME BANK NAME AREA ADDRESS PIN CODE E Mail 1 AMETHI JAGDISHPUR eVIJAYA BANK RURAL LUCKNOW-SULTANPUR ROAD, JAGDISHPUR, SULTANPUR OPP.BLOCK OFFICE 227809 [email protected] 2 RAE BARELI AIHAR eDENA BANK RURAL OPP.SRI GANESH NR RAIL COACH FACTORY 229121 [email protected] 3 RAE BARELI ATARHAR BANK OF BARODA RURAL VILL PO ATARHAR DIST RAEBARELI 229210 [email protected] 4 RAE BARELI BACHHRAWAN BANK OF BARODA SEMI URBAN STATION ROAD BACHHRAWAN,RAEBARELI 229301 [email protected] 5 RAE BARELI BAHAI BANK OF BARODA RURAL VILL PO BAHAI, KANPUR ALLAHABAD ROAD, RAEBAR 229203 [email protected] 6 RAE BARELI BAINTI BANK OF BARODA RURAL VPO BAINTI,BHAWANIGARH SURAJPUR ROAD,UP 229308 [email protected] 7 RAE BARELI BEDAROO BANK OF BARODA RURAL BHAWANIGARH CHAURAHA, P.SHIVALI,T.MAHARAJGAN 229308 [email protected] 8 RAE BARELI BHOJPUR BANK OF BARODA RURAL VILL. & P.O. BHOJPUR, DISTT. RAEBARELI 229205 [email protected] 9 RAE BARELI BIRNAWAN BANK OF BARODA SEMI URBAN VILL BIRNAWA, POST BIR NAWA, AMETHI,UP 229307 [email protected] 10 RAE BARELI CHHIVLAHA BANK OF BARODA RURAL VPO CHHIVLAHA, NEAR PURE PANDEY, BHUPGANJ 229216 [email protected] 11 RAE BARELI DALMAU BANK OF BARODA RURAL DALMAU 229207 [email protected] 12 RAE BARELI DARIYAPUR BANK OF BARODA RURAL DARIYAPUR PO. MUNSIGANJ, 229001 [email protected] 13 RAE BARELI DEDAUR BANK OF BARODA RURAL VILL+PO-DEDAUR 0 229001 [email protected] 14 RAE BARELI DEEH BANK OF BARODA SEMI URBAN B/O DEEH, RAEBARELI 229310 [email protected] 15 RAE BARELI DHARAI BANK OF BARODA RURAL VILL DHARAI, PO SISANI DIST RAEBARELI, UP 229129 [email protected] 16 RAE BARELI FGIET, RAE BARELI BANK OF BARODA URBAN RATAPUR CHAURAH, RAE BAREILLY, UP 229001 [email protected] 17 RAE BARELI GEGASON-CROS BANK OF BARODA RURAL VILL. -

S.No Reg.No Company Name
LIST OF DEFAULTING COMPANIES IN UTTAR PRADESH S.No Reg.No Company Name 1 3 MUIR MILLS CO LTD 2 11 TUNDLA COOPERATIVE STORES LTD 3 12 BHARATVARSHIYA NATIONAL ASSOCIATION 4 13 MEERUT LAW CHAMBERS CO LTD 5 14 WHEELER CLUB LTD. 6 15 BRUSHWARE LTD 7 17 UPPAR INDIA CHAMBERS OF COMMERCE 8 19 CAWNPORE SUGAR WORKS LTD. 9 23 DEHRADUN TEA CO LTD 10 29 CAWNPORE CLUB LTD. 11 31 MURRAY & CO PVT LTD 12 32 SHAHJAHANPUR CLUB PVT LTD 13 33 MAHOMED BAGH CLUB LTD. 14 40 DEHRA DUN CLUB 15 51 ALLAHABAD MILLING CO PVT LTD 16 52 BENARAS BANK LIMITED 17 53 LEGAL PRACTITIONERS ASSOCIATION LTD 18 54 VAISHYA FLOUR & GINNING MILLS CO LTD 19 58 CHAMPARAN SUGAR CO. LTD. 20 76 CHATURVEDI MILLS CO LTD 21 78 UTTAR PRADESH DEVELOPMENT SYSTEMS CORPN LTD 22 84 THE BAREILLY COLL.STAFF P.F.ASSC.LTD. BAREILLY 23 85 NEWSPAPERS LTD. 24 88 UTTAR PRADESH CHALCHITRA NIGAM LTD 25 93 CO-OPERATIVE CO LTD 26 94 SHRI VIKRAM COTTON MILLS LTD 27 98 FORD & MECDONALD PVT LTD 28 115 FRIENDS GLASS WORKS CO LTD 29 118 GORAKHPUR CLUB LIMITED 30 130 AGRA CLUB LTD. 31 135 BUDAUN BAR BUILDING PVT LTD 32 145 CAWNPORE UNION CLUB LIMITED 33 149 J.N.COCOLAS AND CO. PVT LTD 34 152 INDIAN PRESS PVT LTD 35 162 BRITISH INDIA CORPN LTD (11-06-81) 36 169 SC 37 208 U P GLASS LTD 38 230 GANGA GLASS WORKS PVT LTD 39 232 PADRAUNA RAJ KRISHNA SUGAR WORKS LTD 40 242 ATHERTON WEST & CO LTD 41 243 R.