Fossil Creek Native Fish Restoration Project Final Project Report, November, 2005
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Historical Geomorphology of the Verde River
Historical Geomorphology of the Verde River by Philip A. Pearthree Arizona Geological Survey Open-File Report 96-13 June, 1996 Arizona Geological Survey 416 W. Congress, Suite #100, Tucson, Arizona 85701 Research conducted in cooperation with CH2MHILL as part of the basic data collection for assessment of river navigability in Arizona at the time of Statehood (1912 J. Funding was provided by the Arizona State Land Department This report is preliminary and has not been edited or reviewed for conformity with Arizona Geological Survey standards Introduction The Verde River drainage is a major river system that heads in Big Chino Valley in north central Arizona, flows generally southeast through the rugged terrain of central Arizona, and empties into the Salt River east of the Phoenix metropolitan area. It is unusual in Arizona because much the main channel of the Verde River is perennial for much its length. Data summarized in this report were gathered to aid in the assessment of the navigability of the Verde River in February, 1912, when Arizona became a State. These investigations were conducted in cooperation with CH2MHill and were funded by the Arizona State Land Department. The purposes of this report are to (1) outline the geologic and geomorphic framework of the Verde River, (2) to describe the physical character of the channel of the Verde River, and (3) to evaluate how channel morphology and position have changed in the past century. Physiography, Geology, and General Geomorphology of the River The Verde River heads in and flows through the rugged highlands and valleys of central Arizona (Figure 1). -

Gila Topminnow Revised Recovery Plan December 1998
GILA TOPMINNOW, Poeciliopsis occidentalis occidentalis, REVISED RECOVERY PLAN (Original Approval: March 15, 1984) Prepared by David A. Weedman Arizona Game and Fish Department Phoenix, Arizona for Region 2 U.S. Fish and Wildlife Service Albuquerque, New Mexico December 1998 Approved: Regional Director, U.S. Fish and Wildlife Service Date: Gila Topminnow Revised Recovery Plan December 1998 DISCLAIMER Recovery plans delineate reasonable actions required to recover and protect the species. The U.S. Fish and Wildlife Service (Service) prepares the plans, sometimes with the assistance of recovery teams, contractors, State and Federal Agencies, and others. Objectives are attained and any necessary funds made available subject to budgetary and other constraints affecting the parties involved, as well as the need to address other priorities. Time and costs provided for individual tasks are estimates only, and not to be taken as actual or budgeted expenditures. Recovery plans do not necessarily represent the views nor official positions or approval of any persons or agencies involved in the plan formulation, other than the Service. They represent the official position of the Service only after they have been signed by the Regional Director or Director as approved. Approved recovery plans are subject to modification as dictated by new findings, changes in species status, and the completion of recovery tasks. ii Gila Topminnow Revised Recovery Plan December 1998 ACKNOWLEDGMENTS Original preparation of the revised Gila topminnow Recovery Plan (1994) was done by Francisco J. Abarca 1, Brian E. Bagley, Dean A. Hendrickson 1 and Jeffrey R. Simms 1. That document was modified to this current version and the work conducted by those individuals is greatly appreciated and now acknowledged. -

June 2021 Arizona Office of Tourism Monthly State Parks Visitation Report
June 2021 Arizona Office of Tourism Monthly State Parks Visitation Report Arizona State Park Visitation June June 2021 2020 YTD State Park % Chg 2021 2020 YTD YTD % Chg Alamo Lake SP 1,237 1,924 -35.7% 48,720 46,871 3.9% Buckskin Mountain SP 11,092 9,292 19.4% 49,439 45,627 8.4% Catalina SP 6,598 2,420 172.6% 154,763 156,234 -0.9% Cattail Cove SP 10,138 14,908 -32.0% 52,221 67,256 -22.4% Colorado River SHP 167 57 193.0% 2,589 6,220 -58.4% Dead Horse Ranch SP 17,142 18,748 -8.6% 126,799 120,044 5.6% Fool Hollow Lake RA 16,380 25,104 -34.8% 55,360 63,341 -12.6% Fort Verde SHP 706 521 35.5% 4,672 2,654 76.0% Granite Mountain Hotshots MSP 1,076 1,380 -22.0% 11,778 14,874 -20.8% Homolovi SP 4,032 1,982 103.4% 22,007 11,470 91.9% Jerome SHP 4,099 1,991 105.9% 21,789 14,109 54.4% Kartchner Caverns SP 7,776 2,045 280.2% 44,020 57,663 -23.7% Lake Havasu SP 66,040 74,493 -11.3% 241,845 337,920 -28.4% Lost Dutchman SP 4,198 3,651 15.0% 122,844 132,399 -7.2% Lyman Lake SP 11,169 10,625 5.1% 30,755 38,074 -19.2% McFarland SHP 166 0 1,213 2,942 -58.8% Oracle SP 270 486 -44.4% 6,994 9,191 -23.9% Patagonia Lake SP 25,058 30,706 -18.4% 118,311 126,873 -6.7% Picacho Peak SP 1,893 1,953 -3.1% 64,084 68,800 -6.9% Red Rock SP 12,963 6,118 111.9% 56,356 33,228 69.6% Riordan Mansion SHP 777 0 2,188 2,478 -11.7% River Island SP 2,623 3,004 -12.7% 18,089 19,411 -6.8% Roper Lake SP 8,049 12,394 -35.1% 52,924 49,738 6.4% Slide Rock SP 54,922 33,491 64.0% 231,551 110,781 109.0% Tombstone Courthouse SHP 2,875 1,465 96.2% 18,261 16,394 11.4% Tonto Natural Bridge SP 8,146 7,161 13.8% 54,950 36,557 50.3% Tubac Presidio SHP 261 117 123.1% 4,240 2,923 45.1% Yuma Territorial Prison SHP 2,206 1,061 107.9% 28,672 26,505 8.2% Total All Parks 282,059 267,097 5.6% 1,647,434 1,620,577 1.7% Note: Dankworth Pond SP data is included in Roper Lake SP, Sonoita Creek SNA is included in Patagonia Lake SP and Verde River Greenway SNA is included in Dead Horse Ranch SP. -

Fossil Creek Hydrology & Travertine Geomorphology
Fossil Creek Hydrology & Travertine Geomorphology FERC Project No. 2069-003 Arizona Childs and Irving Hydropower License Prepared For: Arizona Public Service Company Phoenix, Arizona Prepared By: W.L. Bouchard & Associates, Inc. Phoenix, Arizona June 25, 1998 Fossil Creek Hydrology & Travertine Geomorphology Abstract Fossil Creek is a tributary of the Verde River in Gila and Yavapai Counties in Arizona. Stream flow is composed of a 43 cubic foot per second (cfs) base flow from Fossil Springs (a perennial source of spring flow to the creek) and runoff from precipitation which frequently occurs as destructive flash floods. These sources form 77% and 23% of the flow respectively. Fossil Springs water is supersaturated with calcium carbonate and carbon dioxide and thus tends to precipitate travertine in the upper 4 mile reach of this 14 mile stream. Travertine deposition effects stream morphology. Fossil Creek is a "flashy" stream that frequently conveys large volumes of water very quickly. Significant floods that overflow the low flow channel banks and transport significant quantities of sediment and debris Occur about every other year. These frequent flood flows tend to alter travertine deposition. Periodically, Fossil Creek is subject to very large destructive floods that significantly alter stream morphology. Arizona Public Service Company (APS) owns and operates the Childs & Irving Hydroelectric plants on Fossil Creek. The plants withdraw the 43 cfs base flow about 0.2 miles below the springs for hydropower generation. In 1992, APS proposed to the Federal Energy Regulatory Commission (FERC) to increase minimum flow releases to 10 cfs in the Irving reach and 5 cfs in the Childs reach as part of their application for relicensing. -

Native Fish Restoration in Redrock Canyon
U.S. Department of the Interior Bureau of Reclamation Final Environmental Assessment Phoenix Area Office NATIVE FISH RESTORATION IN REDROCK CANYON U.S. Department of Agriculture Forest Service Southwestern Region Coronado National Forest Santa Cruz County, Arizona June 2008 Bureau of Reclamation Finding of No Significant Impact U.S. Forest Service Finding of No Significant Impact Decision Notice INTRODUCTION In accordance with the National Environmental Policy Act of 1969 (Public Law 91-190, as amended), the Bureau of Reclamation (Reclamation), as the lead Federal agency, and the Forest Service, U.S. Fish and Wildlife Service (FWS), and Arizona Game and Fish Department (AGFD), as cooperating agencies, have issued the attached final environmental assessment (EA) to disclose the potential environmental impacts resulting from construction of a fish barrier, removal of nonnative fishes with the piscicide antimycin A and/or rotenone, and restoration of native fishes and amphibians in Redrock Canyon on the Coronado National Forest (CNF). The Proposed Action is intended to improve the recovery status of federally listed fish and amphibians (Gila chub, Gila topminnow, Chiricahua leopard frog, and Sonora tiger salamander) and maintain a healthy native fishery in Redrock Canyon consistent with the CNF Plan and ongoing Endangered Species Act (ESA), Section 7(a)(2), consultation between Reclamation and the FWS. BACKGROUND The Proposed Action is part of a larger program being implemented by Reclamation to construct a series of fish barriers within the Gila River Basin to prevent the invasion of nonnative fishes into high-priority streams occupied by imperiled native fishes. This program is mandated by a FWS biological opinion on impacts of Central Arizona Project (CAP) water transfers to the Gila River Basin (FWS 2008a). -
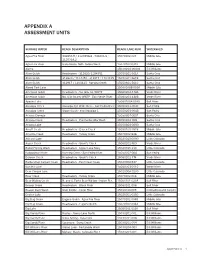
Appendix a Assessment Units
APPENDIX A ASSESSMENT UNITS SURFACE WATER REACH DESCRIPTION REACH/LAKE NUM WATERSHED Agua Fria River 341853.9 / 1120358.6 - 341804.8 / 15070102-023 Middle Gila 1120319.2 Agua Fria River State Route 169 - Yarber Wash 15070102-031B Middle Gila Alamo 15030204-0040A Bill Williams Alum Gulch Headwaters - 312820/1104351 15050301-561A Santa Cruz Alum Gulch 312820 / 1104351 - 312917 / 1104425 15050301-561B Santa Cruz Alum Gulch 312917 / 1104425 - Sonoita Creek 15050301-561C Santa Cruz Alvord Park Lake 15060106B-0050 Middle Gila American Gulch Headwaters - No. Gila Co. WWTP 15060203-448A Verde River American Gulch No. Gila County WWTP - East Verde River 15060203-448B Verde River Apache Lake 15060106A-0070 Salt River Aravaipa Creek Aravaipa Cyn Wilderness - San Pedro River 15050203-004C San Pedro Aravaipa Creek Stowe Gulch - end Aravaipa C 15050203-004B San Pedro Arivaca Cienega 15050304-0001 Santa Cruz Arivaca Creek Headwaters - Puertocito/Alta Wash 15050304-008 Santa Cruz Arivaca Lake 15050304-0080 Santa Cruz Arnett Creek Headwaters - Queen Creek 15050100-1818 Middle Gila Arrastra Creek Headwaters - Turkey Creek 15070102-848 Middle Gila Ashurst Lake 15020015-0090 Little Colorado Aspen Creek Headwaters - Granite Creek 15060202-769 Verde River Babbit Spring Wash Headwaters - Upper Lake Mary 15020015-210 Little Colorado Babocomari River Banning Creek - San Pedro River 15050202-004 San Pedro Bannon Creek Headwaters - Granite Creek 15060202-774 Verde River Barbershop Canyon Creek Headwaters - East Clear Creek 15020008-537 Little Colorado Bartlett Lake 15060203-0110 Verde River Bear Canyon Lake 15020008-0130 Little Colorado Bear Creek Headwaters - Turkey Creek 15070102-046 Middle Gila Bear Wallow Creek N. and S. Forks Bear Wallow - Indian Res. -

Synthesis of Upper Verde River Research and Monitoring 1993-2008
Chapter 8 A Preliminary View of Water Quality Conditions of the Upper Verde River Alvin L. Medina Introduction Stream water temperatures are of general interest because of interactive ef- fects among physical, biological, and chemical parameters of water chemistry (Langford 1990). Water temperature regimes dictate the types of aquatic flora and fauna present within the aquatic system, as well as influence the system’s susceptibility to parasites and disease. These regimes are commonly noted in critical habitat designations as potentially limiting to native fish populations of Southwestern streams (Federal Register 2007). Temperatures that approach the upper thermal tolerances of Southwest native fishes have been noted in Arizona streams (Deacon and Minckley 1974; USDI Geological Survey 2005). Of partic- ular interest are water temperatures for the UVR where spikedace (Meda fulgida) is imperiled. Recent fishery studies (Carveth and others 2006) suggest that na- tive fishes are sensitive to annual and large temperature fluctuations. Reduced growth rates have been reported for some species (Widmer and others 2006). The relationships between desert fishes and water temperature are unclear, especially given the assumption that they should be capable of acclimating to hot and cold temperatures common to the Southwest. State water managers rely on monitoring data to establish and validate water quality standards (Arizona Department of Environmental Quality 2002, 2007a, 2007b). Within the study area, the Arizona Department of Environmental Quality (Arizona Department of Environmental Quality 2007b) listed the headwater sec- tion of the UVR between Granite Creek and Hell Canyon as sufficiently high to support all uses attaining all uses, while the section between Perkinsville to below Camp Verde was listed as “impaired” because of sediment and turbidity. -

Annual Reportarizona YEARS 40…Of Conserving Land and Water to Benefit People and Nature
2006 annual reportArizona YEARS 40…of conserving land and water to benefit people and nature In 1966 a group of conservation-minded citizens raised money to buy the Patagonia-Sonoita Creek Preserve, and The Nature Conservancy in Arizona was born. Over the years that followed, nature preserves throughout the state were purchased by or donated to the Conservancy. They had a common focus: protecting water and VISION: We will ensure freshwater sources are restoring the health of the land. secure and sustainable in order to support our growing population and the rich diversity of life This 40th annual report documents the natural evolution of an that depends on fresh water to thrive. We will work organization whose mission calls upon us to preserve the diversity with water users, providers and those who depend on growth to create the incentives and limits that of life on Earth. We have come to cherish Arizona’s rich biological will guide future growth and create well-planned heritage. We have learned about our vital connections with communities in the face of uncertainty created by neighboring states and other countries through a system of similar securing our future global climate change. habitats or ecoregions. We are beginning to understand how special places are vulnerable to changes that occur many miles away. And, we water now know that change can be friend and foe. Today we are working at an unprecedented scale, on critical issues not recognized 40 years ago, such as Upper San Pedro Partnership Verde River Greenway With the added urgency of the first recorded no- The Conservancy sponsored field trips and provided global warming and the decline in forest health. -

(Central Arizona) GEOSPHERE
Research Paper GEOSPHERE Incision history of the Verde Valley region and implications for uplift of the Colorado Plateau (central Arizona) 1 2 2 GEOSPHERE; v. 14, no. 4 Richard F. Ott , Kelin X. Whipple , and Matthijs van Soest 1Department of Earth Sciences, ETH Zurich, Sonneggstrasse 5, 8092 Zurich, Switzerland 2School of Earth and Space Exploration, Arizona State University, 781 S. Terrace Road, Tempe, Arizona 85287, USA https://doi.org/10.1130/GES01640.1 12 figures; 3 tables; 1 supplemental file ABSTRACT et al., 2008; Moucha et al., 2009; Huntington et al., 2010; Liu and Gurnis, 2010; Flowers and Farley, 2012; Crow et al., 2014; Darling and Whipple, 2015; Karl- CORRESPONDENCE: richard .ott1900@ gmail .com The record of Tertiary landscape evolution preserved in Arizona’s transition strom et al., 2017). As part of this debate, the incision of the Mogollon Rim, zone presents an independent opportunity to constrain the timing of Colo the southwestern edge of the Colorado Plateau (Fig. 1), is not well constrained CITATION: Ott, R.F., Whipple, K.X., and van Soest, rado Plateau uplift and incision. We study this record of landscape evolution in the literature, and disparate ideas about its formation and incision history M., Incision history of the Verde Valley region and implications for uplift of the Colorado Plateau by mapping Tertiary sediments, volcanic deposits, and the erosional uncon have been proposed (Peirce et al., 1979; Lindberg, 1986; Elston and Young, ( central Ari zona): Geosphere, v. 14, no. 4, p. 1690– formity at their base, 40Ar/39Ar dating of basaltic lava flows in key locations, and 1991; Holm, 2001). -

Desert Sucker
Scientific Name Common Name Catostomus clarki desert sucker Bison code 010500 ____________________________________________________________ Official status Endemism ________________________ State AZ: threatened Colorado River Basin _______________________ Status/threats Introduced species and habitat alteration Distribution Presently occurs in the Virgin, Bill Williams, Salt, Verde, San Pedro, Blue, and Gila Rivers in Arizona. Occurs only in the Gila River headwaters in New Mexico and in the San Francisco River. Habitat Occupies low to high gradient riffles (0.5 to 2.0 % gradient) over pebble to cobble substrates containing water velocities of 0.5 to 1.5 cm sec. Preferred temperature of 17.5 C in Virgin River and calculated critical thermal maximums of 32-37 C. Life history and ecology Morphological complex in this species renders taxonomy difficult, however, probably more than one species exists across its range in Arizona and New Mexico. Two forms are apparent in the Virgin River basin, one in the Bill Williams basin and another form in the Bill Williams and Gila basins. Detailed genetic and morphological studies and frequent hybridization between the genera Pantosteus and Catostomus have resulted in Pantosteus being relegated to sub-generic status. Breeding Generally occurs in riffles, however, not specifically studied. Key Habitat Components: Low to high gradient riffles of moderate to swift velocity current, moderate depth (< 0.5 m), and over pebble to cobble-boulder substrate. Also in pools with moderate current. Breeding season Late winter (Jan-Feb) to early spring (March-April). Grazing effects The widespread, adaptable, and ubiquitous in addition to its generally low to high gradient riffle habitat of cobble to boulder substrates renders grazing of little potential impact to this species. -

Download Paddle Map 3
Verde River Paddle Trail Highway 89A Bridge to Beasley Flat Cover Photo Courtesy of Susun McCulla Welcome to The Verde River Paddle Trail Highway 89A Bridge to Beasley Flat This paddle guide is the result of the vision and dedication of John Parsons. Many heartfelt thanks to John, his wife Susun, and his young boating partner Josh Wheeler for performing the entire field work for this guide. Special thanks to John for his decades of advocacy in support of a healthy Verde River. Photo courtesy of Gary Beverly This section of the Verde River Paddle Trail covers 31 river miles from the Highway 89A Bridgeport Bridge to Beasley Flat. The guide is composed of nine map segments, each with a corresponding descriptive narrative of GPS waypoints. A vicinity map shows how the segment maps provide coverage of each river reach, and a waypoint table provides GPS coordinates for the waypoints used in this guide. Note that much of this section of river is located on private property. Please respect the rights of these property owners during your paddle trip; it is an act of trespass to disembark and enter riverside properties. Plan your river trip accordingly and leave the river on public lands only. The Verde River is a dynamic natural resource. Water levels, channel obstructions and many other factors can change on a daily basis. River recreationists are advised to practice Safe Paddling. 1 Verde River Paddle Trail Highway 89A Bridge to Beasley Flat Safe Paddling • Never paddle alone • Inform friends and/or family of your paddling plans • Check conditions before you go • Dress appropriately for the expected weather • Wear a life jacket • Know your watercraft’s limitations • Paddle within your skill level • Avoid high, swift water • Scout any hazard about which you have doubts. -

The East Verde Is a Rapidly Degrading Stream Flowing Through a Country Of
An archeological reconnaissance of the East Verde River in central Arizona Item Type Thesis-Reproduction (electronic); text Authors Peck, Fred Rawlings,1925- Publisher The University of Arizona. Rights Copyright © is held by the author. Digital access to this material is made possible by the University Libraries, University of Arizona. Further transmission, reproduction or presentation (such as public display or performance) of protected items is prohibited except with permission of the author. Download date 23/09/2021 22:17:51 Link to Item http://hdl.handle.net/10150/191414 FroritespieCe The East Verde is a rapidly degradingstream flowing through a country of high relief. AN AtC HEOLOGICAL RECO ISANCE OF THE Ei$T VERDE RIVER IN CENTPLMIDNA by Fred R. Peck A Thesis submitted to the faculty of the Department of Anthropology in partial fulfillment of the requirements for the degree of M$TER OF ARTS in the Graduate College, University of Arizona l96 Approved: Director of T is Y Date /95 This thesis has been submitted in partial fulfillment of requirements for an advanced degree at the University of Arizona and is deposited in the Library to be made available to borrowers under rules of the Library. Brief quotations from this thesis are allowable without special permission, provided that accurate acknowledgment of source is made. Requests for permission for extended quotation from or repro- duction of this manuscript in whole or in part may be granted by the head of the major department or the dean of the Graduate College when in their judgment the proposed use of the material is in the interests of scholarship.